FGF-signalling in the differentiation of mouse ES cells towards ...
FGF-signalling in the differentiation of mouse ES cells towards ...
FGF-signalling in the differentiation of mouse ES cells towards ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
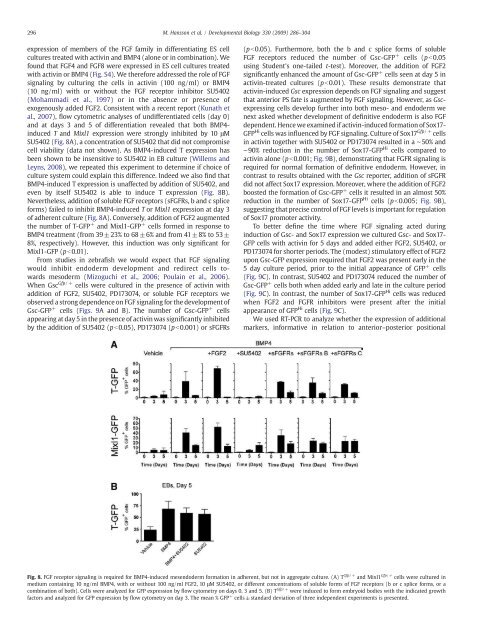
296 M. Hansson et al. / Developmental Biology 330 (2009) 286–304expression <strong>of</strong> members <strong>of</strong> <strong>the</strong> <strong>FGF</strong> family <strong>in</strong> differentiat<strong>in</strong>g <strong>ES</strong> cellcultures treated with activ<strong>in</strong> and BMP4 (alone or <strong>in</strong> comb<strong>in</strong>ation). Wefound that <strong>FGF</strong>4 and <strong>FGF</strong>8 were expressed <strong>in</strong> <strong>ES</strong> cell cultures treatedwith activ<strong>in</strong> or BMP4 (Fig. S4). We <strong>the</strong>refore addressed <strong>the</strong> role <strong>of</strong> <strong>FGF</strong>signal<strong>in</strong>g by cultur<strong>in</strong>g <strong>the</strong> <strong>cells</strong> <strong>in</strong> activ<strong>in</strong> (100 ng/ml) or BMP4(10 ng/ml) with or without <strong>the</strong> <strong>FGF</strong> receptor <strong>in</strong>hibitor SU5402(Mohammadi et al., 1997) or <strong>in</strong> <strong>the</strong> absence or presence <strong>of</strong>exogenously added <strong>FGF</strong>2. Consistent with a recent report (Kunath etal., 2007), flow cytometric analyses <strong>of</strong> undifferentiated <strong>cells</strong> (day 0)and at days 3 and 5 <strong>of</strong> <strong>differentiation</strong> revealed that both BMP4-<strong>in</strong>duced T and Mixl1 expression were strongly <strong>in</strong>hibited by 10 μMSU5402 (Fig. 8A), a concentration <strong>of</strong> SU5402 that did not compromisecell viability (data not shown). As BMP4-<strong>in</strong>duced T expression hasbeen shown to be <strong>in</strong>sensitive to SU5402 <strong>in</strong> EB culture (Willems andLeyns, 2008), we repeated this experiment to determ<strong>in</strong>e if choice <strong>of</strong>culture system could expla<strong>in</strong> this difference. Indeed we also f<strong>in</strong>d thatBMP4-<strong>in</strong>duced T expression is unaffected by addition <strong>of</strong> SU5402, andeven by itself SU5402 is able to <strong>in</strong>duce T expression (Fig. 8B).Never<strong>the</strong>less, addition <strong>of</strong> soluble <strong>FGF</strong> receptors (s<strong>FGF</strong>Rs, b and c spliceforms) failed to <strong>in</strong>hibit BMP4-<strong>in</strong>duced T or Mixl1 expression at day 3<strong>of</strong> adherent culture (Fig. 8A). Conversely, addition <strong>of</strong> <strong>FGF</strong>2 augmented<strong>the</strong> number <strong>of</strong> T-GFP + and Mixl1-GFP + <strong>cells</strong> formed <strong>in</strong> response toBMP4 treatment (from 39±23% to 68 ±6% and from 41±8% to 53±8%, respectively). However, this <strong>in</strong>duction was only significant forMixl1-GFP (pb0.01).From studies <strong>in</strong> zebrafish we would expect that <strong>FGF</strong> signal<strong>in</strong>gwould <strong>in</strong>hibit endoderm development and redirect <strong>cells</strong> <strong>towards</strong>mesoderm (Mizoguchi et al., 2006; Poula<strong>in</strong> et al., 2006).When Gsc Gfp/+ <strong>cells</strong> were cultured <strong>in</strong> <strong>the</strong> presence <strong>of</strong> activ<strong>in</strong> withaddition <strong>of</strong> <strong>FGF</strong>2, SU5402, PD173074, or soluble <strong>FGF</strong> receptors weobserved a strong dependence on <strong>FGF</strong> signal<strong>in</strong>g for <strong>the</strong> development <strong>of</strong>Gsc-GFP + <strong>cells</strong> (Figs. 9A and B). The number <strong>of</strong> Gsc-GFP + <strong>cells</strong>appear<strong>in</strong>g at day 5 <strong>in</strong> <strong>the</strong> presence <strong>of</strong> activ<strong>in</strong> was significantly <strong>in</strong>hibitedby <strong>the</strong> addition <strong>of</strong> SU5402 (pb0.05), PD173074 (pb0.001) or s<strong>FGF</strong>Rs(pb0.05). Fur<strong>the</strong>rmore, both <strong>the</strong> b and c splice forms <strong>of</strong> soluble<strong>FGF</strong> receptors reduced <strong>the</strong> number <strong>of</strong> Gsc-GFP + <strong>cells</strong> (p b0.05us<strong>in</strong>g Student's one-tailed t-test). Moreover, <strong>the</strong> addition <strong>of</strong> <strong>FGF</strong>2significantly enhanced <strong>the</strong> amount <strong>of</strong> Gsc-GFP + <strong>cells</strong> seen at day 5 <strong>in</strong>activ<strong>in</strong>-treated cultures (pb0.01). These results demonstrate thatactiv<strong>in</strong>-<strong>in</strong>duced Gsc expression depends on <strong>FGF</strong> signal<strong>in</strong>g and suggestthat anterior PS fate is augmented by <strong>FGF</strong> signal<strong>in</strong>g. However, as Gscexpress<strong>in</strong>g<strong>cells</strong> develop fur<strong>the</strong>r <strong>in</strong>to both meso- and endoderm wenext asked whe<strong>the</strong>r development <strong>of</strong> def<strong>in</strong>itive endoderm is also <strong>FGF</strong>dependent. Hence we exam<strong>in</strong>ed if activ<strong>in</strong>-<strong>in</strong>duced formation <strong>of</strong> Sox17-GFP Hi <strong>cells</strong> was <strong>in</strong>fluenced by <strong>FGF</strong> signal<strong>in</strong>g. Culture <strong>of</strong> Sox17 Gfp/+ <strong>cells</strong><strong>in</strong> activ<strong>in</strong> toge<strong>the</strong>r with SU5402 or PD173074 resulted <strong>in</strong> a ∼50% and∼90% reduction <strong>in</strong> <strong>the</strong> number <strong>of</strong> Sox17-GFP Hi <strong>cells</strong> compared toactiv<strong>in</strong> alone (pb0.001; Fig. 9B), demonstrat<strong>in</strong>g that <strong>FGF</strong>R signal<strong>in</strong>g isrequired for normal formation <strong>of</strong> def<strong>in</strong>itive endoderm. However, <strong>in</strong>contrast to results obta<strong>in</strong>ed with <strong>the</strong> Gsc reporter, addition <strong>of</strong> s<strong>FGF</strong>Rdid not affect Sox17 expression. Moreover, where <strong>the</strong> addition <strong>of</strong> <strong>FGF</strong>2boosted <strong>the</strong> formation <strong>of</strong> Gsc-GFP + <strong>cells</strong> it resulted <strong>in</strong> an almost 50%reduction <strong>in</strong> <strong>the</strong> number <strong>of</strong> Sox17-GFP Hi <strong>cells</strong> (pb0.005; Fig. 9B),suggest<strong>in</strong>g that precise control <strong>of</strong> <strong>FGF</strong> levels is important for regulation<strong>of</strong> Sox17 promoter activity.To better def<strong>in</strong>e <strong>the</strong> time where <strong>FGF</strong> signal<strong>in</strong>g acted dur<strong>in</strong>g<strong>in</strong>duction <strong>of</strong> Gsc- and Sox17 expression we cultured Gsc- and Sox17-GFP <strong>cells</strong> with activ<strong>in</strong> for 5 days and added ei<strong>the</strong>r <strong>FGF</strong>2, SU5402, orPD173074 for shorter periods. The (modest) stimulatory effect <strong>of</strong> <strong>FGF</strong>2upon Gsc-GFP expression required that <strong>FGF</strong>2 was present early <strong>in</strong> <strong>the</strong>5 day culture period, prior to <strong>the</strong> <strong>in</strong>itial appearance <strong>of</strong> GFP + <strong>cells</strong>(Fig. 9C). In contrast, SU5402 and PD173074 reduced <strong>the</strong> number <strong>of</strong>Gsc-GFP + <strong>cells</strong> both when added early and late <strong>in</strong> <strong>the</strong> culture period(Fig. 9C). In contrast, <strong>the</strong> number <strong>of</strong> Sox17-GFP Hi <strong>cells</strong> was reducedwhen <strong>FGF</strong>2 and <strong>FGF</strong>R <strong>in</strong>hibitors were present after <strong>the</strong> <strong>in</strong>itialappearance <strong>of</strong> GFP Hi <strong>cells</strong> (Fig. 9C).We used RT-PCR to analyze whe<strong>the</strong>r <strong>the</strong> expression <strong>of</strong> additionalmarkers, <strong>in</strong>formative <strong>in</strong> relation to anterior–posterior positionalFig. 8. <strong>FGF</strong> receptor signal<strong>in</strong>g is required for BMP4-<strong>in</strong>duced mesendoderm formation <strong>in</strong> adherent, but not <strong>in</strong> aggregate culture. (A) T Gfp/+ and Mixl1 Gfp/+ <strong>cells</strong> were cultured <strong>in</strong>medium conta<strong>in</strong><strong>in</strong>g 10 ng/ml BMP4, with or without 100 ng/ml <strong>FGF</strong>2, 10 μM SU5402, or different concentrations <strong>of</strong> soluble forms <strong>of</strong> <strong>FGF</strong> receptors (b or c splice forms, or acomb<strong>in</strong>ation <strong>of</strong> both). Cells were analyzed for GFP expression by flow cytometry on days 0, 3 and 5. (B) T Gfp/+ were <strong>in</strong>duced to form embryoid bodies with <strong>the</strong> <strong>in</strong>dicated growthfactors and analyzed for GFP expression by flow cytometry on day 3. The mean % GFP + <strong>cells</strong> ±standard deviation <strong>of</strong> three <strong>in</strong>dependent experiments is presented.