FGF-signalling in the differentiation of mouse ES cells towards ...
FGF-signalling in the differentiation of mouse ES cells towards ...
FGF-signalling in the differentiation of mouse ES cells towards ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
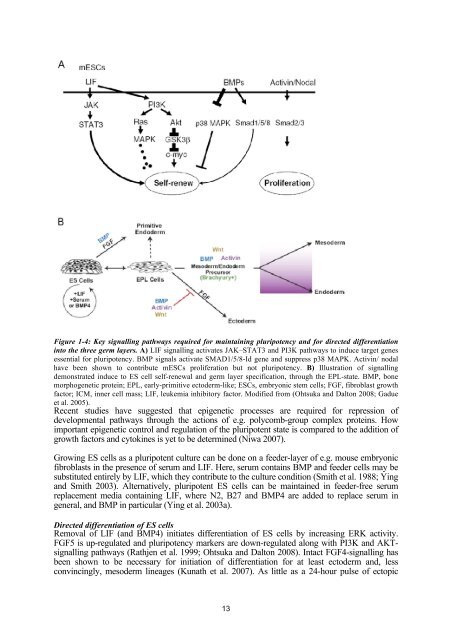
Figure 1-4: Key <strong>signall<strong>in</strong>g</strong> pathways required for ma<strong>in</strong>ta<strong>in</strong><strong>in</strong>g pluripotency and for directed <strong>differentiation</strong><strong>in</strong>to <strong>the</strong> three germ layers. A) LIF <strong>signall<strong>in</strong>g</strong> activates JAK–STAT3 and PI3K pathways to <strong>in</strong>duce target genesessential for pluripotency. BMP signals activate SMAD1/5/8-Id gene and suppress p38 MAPK. Activ<strong>in</strong>/ nodalhave been shown to contribute m<strong>ES</strong>Cs proliferation but not pluripotency. B) Illustration <strong>of</strong> <strong>signall<strong>in</strong>g</strong>demonstrated <strong>in</strong>duce to <strong>ES</strong> cell self-renewal and germ layer specification, through <strong>the</strong> EPL-state. BMP, bonemorphogenetic prote<strong>in</strong>; EPL, early-primitive ectoderm-like; <strong>ES</strong>Cs, embryonic stem <strong>cells</strong>; <strong>FGF</strong>, fibroblast growthfactor; ICM, <strong>in</strong>ner cell mass; LIF, leukemia <strong>in</strong>hibitory factor. Modified from (Ohtsuka and Dalton 2008; Gadueet al. 2005).Recent studies have suggested that epigenetic processes are required for repression <strong>of</strong>developmental pathways through <strong>the</strong> actions <strong>of</strong> e.g. polycomb-group complex prote<strong>in</strong>s. Howimportant epigenetic control and regulation <strong>of</strong> <strong>the</strong> pluripotent state is compared to <strong>the</strong> addition <strong>of</strong>growth factors and cytok<strong>in</strong>es is yet to be determ<strong>in</strong>ed (Niwa 2007).Grow<strong>in</strong>g <strong>ES</strong> <strong>cells</strong> as a pluripotent culture can be done on a feeder-layer <strong>of</strong> e.g. <strong>mouse</strong> embryonicfibroblasts <strong>in</strong> <strong>the</strong> presence <strong>of</strong> serum and LIF. Here, serum conta<strong>in</strong>s BMP and feeder <strong>cells</strong> may besubstituted entirely by LIF, which <strong>the</strong>y contribute to <strong>the</strong> culture condition (Smith et al. 1988; Y<strong>in</strong>gand Smith 2003). Alternatively, pluripotent <strong>ES</strong> <strong>cells</strong> can be ma<strong>in</strong>ta<strong>in</strong>ed <strong>in</strong> feeder-free serumreplacement media conta<strong>in</strong><strong>in</strong>g LIF, where N2, B27 and BMP4 are added to replace serum <strong>in</strong>general, and BMP <strong>in</strong> particular (Y<strong>in</strong>g et al. 2003a).Directed <strong>differentiation</strong> <strong>of</strong> <strong>ES</strong> <strong>cells</strong>Removal <strong>of</strong> LIF (and BMP4) <strong>in</strong>itiates <strong>differentiation</strong> <strong>of</strong> <strong>ES</strong> <strong>cells</strong> by <strong>in</strong>creas<strong>in</strong>g ERK activity.<strong>FGF</strong>5 is up-regulated and pluripotency markers are down-regulated along with PI3K and AKT<strong>signall<strong>in</strong>g</strong>pathways (Rathjen et al. 1999; Ohtsuka and Dalton 2008). Intact <strong>FGF</strong>4-<strong>signall<strong>in</strong>g</strong> hasbeen shown to be necessary for <strong>in</strong>itiation <strong>of</strong> <strong>differentiation</strong> for at least ectoderm and, lessconv<strong>in</strong>c<strong>in</strong>gly, mesoderm l<strong>in</strong>eages (Kunath et al. 2007). As little as a 24-hour pulse <strong>of</strong> ectopic13