Public Health and Communicable Diseases - SA Health - SA.Gov.au
Public Health and Communicable Diseases - SA Health - SA.Gov.au Public Health and Communicable Diseases - SA Health - SA.Gov.au
H5N1 is not yet readily transmissible from humanto human. The body temperature of birds is higherthan that of humans, at around 40 o C, and it has beensuggested that H5N1 has not yet adapted to the humanbody temperature, particularly in the upper airways.New research has also shown that H5N1 virus bindsto receptors which are present in the lungs rather thanin the upper airways 8 . Viral proliferation and sheddingthus occurs predominantly in the lower respiratorytract, reducing infectivity of droplets generated from theoropharynx during coughing and sneezing.Human disease associated with the H5N1 ‘bird flu’outbreak has been unusually severe, for reasons whichare not fully understood, though probably involveoverproduction of proinflammatory cytokines andevasion of their antiviral effects 9 . Reported mortalityrates have exceeded 50% which is much higher thanthat observed in seasonal influenza. Although human tohuman transmission has not been documented so far,it is interesting that many cases have occurred amongblood relatives. There have been no cases reportedamong persons involved in culling infected poultry,who could be expected to be at high risk of infection.This suggests that there may be a genetic element tosusceptibility, although this remains speculative.Will H5N1 cause the next pandemic? Never before hasthe emergence of an influenza pandemic been metwith such a high level of surveillance and preparedness.Close cooperation between the World Organisationfor Animal Health (OIE), and WHO, the human WorldHealth Organisation, has resulted in rapid responses tooutbreaks of avian influenza in both animals and humansworldwide. By “stamping out” infections in poultry, theopportunity for the virus to infect and mutate in humanshas been much reduced. Authorities believe it may bepossible to contain H5N1 through global cooperation.So far this has been helped by the low infectivity of thevirus to humans, and the fact that it has not adapted foreasy transmissibility between humans. If this changesor response teams become overwhelmed, then the nextpandemic may occur very soon.Should a pandemic occur, it will be met by anunprecedented public health response. The World HealthOrganisation, and governments in Australia and overseasare working on plans for containment and quarantine,administration of anti-virals and vaccines, maintenanceof essential services, and provision of acute medicalcare for up to one third of the population. Since the lastpandemic, we have developed technology allowing rapidtyping and surveillance of influenza viruses, vaccinesagainst influenza viruses, anti-viral drugs, better infectioncontrol knowledge and equipment, and sophisticatedcommunications to enable spread of information aroundthe world within minutes. However we also haverapid international transport, a much larger population,and communities whose structures have altereddramatically since the last pandemic, all of which willmake transmission of a pandemic virus easier. The rapidglobal spread of SARS illustrated the impact of a newviral respiratory illness that can be easily transmittedfrom human to human in the era of jet travel. The worldwatches with fear, awe, and hope that a killer pandemicwill not occur again.References1. Hinshaw VS, Webster RG. The natural history ofinfluenza A viruses. In: Beare AS, editor. 79-104. Basicand applied influenza research. Boca Raton, Fla., CRCPress, Inc.1982.2. Gerhard W, Mozdanowska K, Zharikova D. Prospectsfor universal influenza virus vaccine. Emerg Infect Dis.2006;12(4):569-74.3. Treanor JJ. Influenza virus. 2060-85. In: Mandell,Douglas, and Bennett’s principles and practice ofinfectious diseases. 6 th ed. Mandell GL, Bennett JE,Dolin R, editors. Philadelphia, Pen; Elsevier ChurchillLivingstone; 2005.4. Webster RG, Bean WJ, Gorman OT, et al. Evolutionand ecology of influenza A viruses. Microbiol Rev.1992;56(1):152-79.5. Tumpey TM, Basler CF, Aguilar PV, et al.Characterization of the reconstructed 1918 Spanishinfluenza pandemic virus. Science. 2005;310:77-80.6. Guan Y, Poon LLM. Cheung CY, et al. H5N1 influenza:a protean pandemic threat. PNAS. 2004;101(21):8156-61.7. Schnurrenberger, PR, Woods GT, Martin RJ. Serologicevidence of human infection with swine influenzavirus. Am Rev Respir Dis. 1970;102:356-361.8. Shinya K, Ebina M, Yamada S, et al. Avian flu:influenza virus receptors in the human airway. Nature.2006;440(7083):435-6.9. Seo SH, Hoffman E, Webster RG. Lethal H5N1influenza viruses escape host anti-viral cytokineresponses. Nat Med. 2002;8(9):950-4.
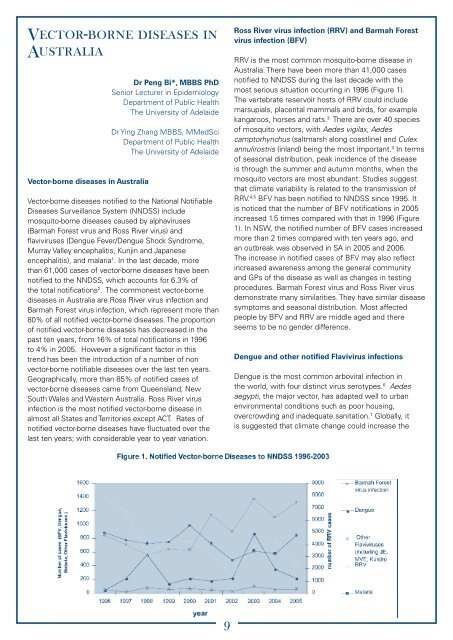
Vector-borne diseases inAustraliaVector-borne diseases in AustraliaDr Peng Bi*, MBBS PhDSenior Lecturer in EpidemiologyDepartment of Public HealthThe University of AdelaideDr Ying Zhang MBBS, MMedSciDepartment of Public HealthThe University of AdelaideVector-borne diseases notified to the National NotifiableDiseases Surveillance System (NNDSS) includemosquito-borne diseases caused by alphaviruses(Barmah Forest virus and Ross River virus) andflaviviruses (Dengue Fever/Dengue Shock Syndrome,Murray Valley encephalitis, Kunjin and Japaneseencephalitis), and malaria 1 . In the last decade, morethan 61,000 cases of vector-borne diseases have beennotified to the NNDSS, which accounts for 6.3% ofthe total notifications 2 . The commonest vector-bornediseases in Australia are Ross River virus infection andBarmah Forest virus infection, which represent more than80% of all notified vector-borne diseases. The proportionof notified vector-borne diseases has decreased in thepast ten years, from 16% of total notifications in 1996to 4% in 2005. However a significant factor in thistrend has been the introduction of a number of nonvector-borne notifiable diseases over the last ten years.Geographically, more than 85% of notified cases ofvector-borne diseases came from Queensland, NewSouth Wales and Western Australia. Ross River virusinfection is the most notified vector-borne disease inalmost all States and Territories except ACT. Rates ofnotified vector-borne diseases have fluctuated over thelast ten years; with considerable year to year variation.Ross River virus infection (RRV) and Barmah Forestvirus infection (BFV)RRV is the most common mosquito-borne disease inAustralia. There have been more than 41,000 casesnotified to NNDSS during the last decade with themost serious situation occurring in 1996 (Figure 1).The vertebrate reservoir hosts of RRV could includemarsupials, placental mammals and birds, for examplekangaroos, horses and rats. 3 There are over 40 speciesof mosquito vectors, with Aedes vigilax, Aedescamptorhynchus (saltmarsh along coastline) and Culexannulirostris (inland) being the most important. 3 In termsof seasonal distribution, peak incidence of the diseaseis through the summer and autumn months, when themosquito vectors are most abundant. Studies suggestthat climate variability is related to the transmission ofRRV. 4,5 BFV has been notified to NNDSS since 1995. Itis noticed that the number of BFV notifications in 2005increased 1.5 times compared with that in 1996 (Figure1). In NSW, the notified number of BFV cases increasedmore than 2 times compared with ten years ago, andan outbreak was observed in SA in 2005 and 2006.The increase in notified cases of BFV may also reflectincreased awareness among the general communityand GPs of the disease as well as changes in testingprocedures. Barmah Forest virus and Ross River virusdemonstrate many similarities. They have similar diseasesymptoms and seasonal distribution. Most affectedpeople by BFV and RRV are middle aged and thereseems to be no gender difference.Dengue and other notified Flavivirus infectionsDengue is the most common arboviral infection inthe world, with four distinct virus serotypes. 6 Aedesaegypti, the major vector, has adapted well to urbanenvironmental conditions such as poor housing,overcrowding and inadequate sanitation. 7 Globally, itis suggested that climate change could increase the
- Page 1 and 2: Edition 4 /2006 South AustraliaPubl
- Page 3: Emphasis on particular goals of pub
- Page 6 and 7: References1. Teutsch SM. Considerat
- Page 10 and 11: number of people living in areas of
- Page 12 and 13: Infections, kidneyand cardiovascula
- Page 14 and 15: living conditions and health status
- Page 16 and 17: Hepatitis C virusinfection in priso
- Page 18 and 19: population). Data collection commen
- Page 20 and 21: 31 Weild AR, Gill ON, Bennett D, Li
- Page 22 and 23: avoidance strategies. To promote po
- Page 24 and 25: cancer among aboriginal women. This
- Page 26 and 27: coverage had dropped by 21% and mum
- Page 28 and 29: CASE STUDY 2Parents’ experiences
- Page 30 and 31: Chlamydia -closing thestable doorDr
- Page 32 and 33: Sexually transmittedinfections in C
- Page 34 and 35: Early treatment of infections is es
- Page 36 and 37: Australian hospitals, the optimum r
- Page 38 and 39: Figure 3: Relationship between new
- Page 40 and 41: While ongoing monitoring and analys
- Page 42 and 43: InfluenzaSouth Australian influenza
- Page 44: Notifiable Diseases in South Austra
Vector-borne diseases inAustraliaVector-borne diseases in AustraliaDr Peng Bi*, MBBS PhDSenior Lecturer in EpidemiologyDepartment of <strong>Public</strong> <strong>Health</strong>The University of AdelaideDr Ying Zhang MBBS, MMedSciDepartment of <strong>Public</strong> <strong>Health</strong>The University of AdelaideVector-borne diseases notified to the National Notifiable<strong>Diseases</strong> Surveillance System (NNDSS) includemosquito-borne diseases c<strong>au</strong>sed by alphaviruses(Barmah Forest virus <strong>and</strong> Ross River virus) <strong>and</strong>flaviviruses (Dengue Fever/Dengue Shock Syndrome,Murray Valley encephalitis, Kunjin <strong>and</strong> Japaneseencephalitis), <strong>and</strong> malaria 1 . In the last decade, morethan 61,000 cases of vector-borne diseases have beennotified to the NNDSS, which accounts for 6.3% ofthe total notifications 2 . The commonest vector-bornediseases in Australia are Ross River virus infection <strong>and</strong>Barmah Forest virus infection, which represent more than80% of all notified vector-borne diseases. The proportionof notified vector-borne diseases has decreased in thepast ten years, from 16% of total notifications in 1996to 4% in 2005. However a significant factor in thistrend has been the introduction of a number of nonvector-borne notifiable diseases over the last ten years.Geographically, more than 85% of notified cases ofvector-borne diseases came from Queensl<strong>and</strong>, NewSouth Wales <strong>and</strong> Western Australia. Ross River virusinfection is the most notified vector-borne disease inalmost all States <strong>and</strong> Territories except ACT. Rates ofnotified vector-borne diseases have fluctuated over thelast ten years; with considerable year to year variation.Ross River virus infection (RRV) <strong>and</strong> Barmah Forestvirus infection (BFV)RRV is the most common mosquito-borne disease inAustralia. There have been more than 41,000 casesnotified to NNDSS during the last decade with themost serious situation occurring in 1996 (Figure 1).The vertebrate reservoir hosts of RRV could includemarsupials, placental mammals <strong>and</strong> birds, for examplekangaroos, horses <strong>and</strong> rats. 3 There are over 40 speciesof mosquito vectors, with Aedes vigilax, Aedescamptorhynchus (saltmarsh along coastline) <strong>and</strong> Culexannulirostris (inl<strong>and</strong>) being the most important. 3 In termsof seasonal distribution, peak incidence of the diseaseis through the summer <strong>and</strong> <strong>au</strong>tumn months, when themosquito vectors are most abundant. Studies suggestthat climate variability is related to the transmission ofRRV. 4,5 BFV has been notified to NNDSS since 1995. Itis noticed that the number of BFV notifications in 2005increased 1.5 times compared with that in 1996 (Figure1). In NSW, the notified number of BFV cases increasedmore than 2 times compared with ten years ago, <strong>and</strong>an outbreak was observed in <strong>SA</strong> in 2005 <strong>and</strong> 2006.The increase in notified cases of BFV may also reflectincreased awareness among the general community<strong>and</strong> GPs of the disease as well as changes in testingprocedures. Barmah Forest virus <strong>and</strong> Ross River virusdemonstrate many similarities. They have similar diseasesymptoms <strong>and</strong> seasonal distribution. Most affectedpeople by BFV <strong>and</strong> RRV are middle aged <strong>and</strong> thereseems to be no gender difference.Dengue <strong>and</strong> other notified Flavivirus infectionsDengue is the most common arboviral infection inthe world, with four distinct virus serotypes. 6 Aedesaegypti, the major vector, has adapted well to urbanenvironmental conditions such as poor housing,overcrowding <strong>and</strong> inadequate sanitation. 7 Globally, itis suggested that climate change could increase the