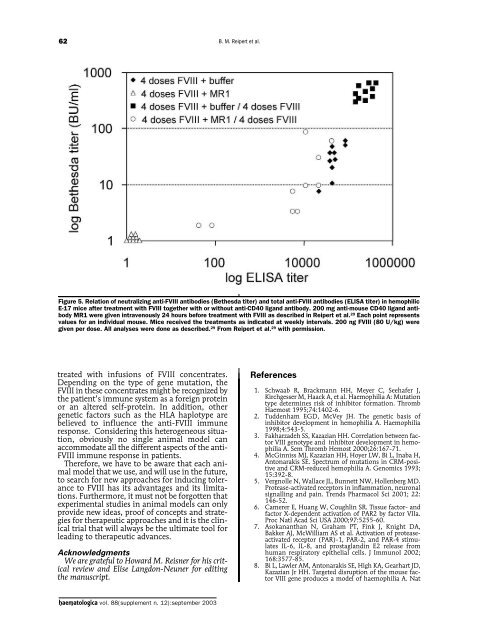
62B. M. Reipert et al.Figure 5. Relation of neutralizing anti-FVIII antibodies (Bethesda titer) and total anti-FVIII antibodies (ELISA titer) in hemophilicE-17 mice after treatment with FVIII together with or without anti-CD40 ligand antibody. 200 mg anti-mouse CD40 ligand antibodyMR1 were given intravenously 24 hours before treatment with FVIII as described in Reipert et al. 29 Each point representsvalues for an individual mouse. Mice received the treatments as indicated at weekly intervals. 200 ng FVIII (80 U/kg) weregiven per dose. All analyses were done as described. 29 From Reipert et al. 29 with permission.treated with infusions of FVIII concentrates.Depending on the type of gene mutation, theFVIII in these concentrates might be recognized bythe patient’s immune system as a foreign proteinor an altered self-protein. In addition, othergenetic factors such as the HLA haplotype arebelieved to influence the anti-FVIII immuneresponse. Considering this heterogeneous situation,obviously no single animal model canaccommodate all the different aspects of the anti-FVIII immune response in patients.Therefore, we have to be aware that each animalmodel that we use, and will use in the future,to search for new approaches for inducing toleranceto FVIII has its advantages and its limitations.Furthermore, it must not be forgotten thatexperimental studies in animal models can onlyprovide new ideas, proof of concepts and strategiesfor therapeutic approaches and it is the clinicaltrial that will always be the ultimate tool forleading to therapeutic advances.AcknowledgmentsWe are grateful to Howard M. Reisner for his criticalreview and Elise Langdon-Neuner for editingthe manuscript.References1. Schwaab R, Brackmann HH, Meyer C, Seehafer J,Kirchgesser M, Haack A, et al. Haemophilia A: Mutationtype determines risk of inhibitor formation. ThrombHaemost 1995;74:1402-6.2. Tuddenham EGD, McVey JH. The genetic basis ofinhibitor development in hemophilia A. Haemophilia1998;4:543-5.3. Fakharzadeh SS, Kazazian HH. Correlation between factorVIII genotype and inhibitor development in hemophiliaA. Sem Thromb Hemost 2000;26:167-71.4. McGinniss MJ, Kazazian HH, Hoyer LW, Bi L, Inaba H,Antonarakis SE. Spectrum of mutations in CRM-positiveand CRM-reduced hemophilia A. Genomics 1993;15:392-8.5. Vergnolle N, Wallace JL, Bunnett NW, Hollenberg MD.Protease-activated receptors in inflammation, neuronalsignalling and pain. Trends Pharmacol Sci 2001; 22:146-52.6. Camerer E, Huang W, Coughlin SR. Tissue factor- andfactor X-dependent activation of PAR2 by factor VIIa.Proc Natl Acad Sci USA 2000;97:5255-60.7. Asokananthan N, Graham PT, Fink J, Knight DA,Bakker AJ, McWilliam AS et al. Activation of proteaseactivatedreceptor (PAR)-1, PAR-2, and PAR-4 stimulatesIL-6, IL-8, and prostaglandin E2 release fromhuman respiratory epithelial cells. J Immunol 2002;168:3577-85.8. Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD,Kazazian Jr HH. Targeted disruption of the mouse factorVIII gene produces a model of haemophilia A. Nathaematologica vol. 88(supplement n. 12):september <strong>2003</strong>
IV International Workshop on Immune Tolerance in Hemophilia63Genet 1995;10:119-21.9. Bi L, Sarkar R, Naas T, Lawler AM, Pain J, Shumaker SL,et al. Further characterization of factor VIII-deficientmice created by gene targeting: RNA and protein studies.Blood 1996;88:3446-50.10. Muchitsch EM, Turecek PL, Zimmermann K, Pichler L,Auer W, Richter G, et al. Phenotypic expression ofmurine hemophilia. Thromb Haemost 1999;82:1371-3.11. Connelly S, Andrews JL, Gallo AM, Kayda DB, Qian J,Hoyer L, et al. Sustained phenotypic correction ofmurine hemophilia A by in vivo gene therapy. Blood1998;91:3273-81.12. Qian J, Borovok M, Bi L, Kazazian Jr HH, Hoyer LW.Inhibitor development and T cell response to humanfactor VIII in murine haemophilia A. Thromb Haemost1999;81:240-4.13. Balague C, Zhou J, Dai Y, Alemany R, Josephs SF,Andreason G, et al. Sustained high-level expression offull-length human factor VIII and restoration of clottingactivity in hemophilic mice using a minimal adenovirusvector. Blood 2000;95:820-8.14. Elder B, Lakich D, Gitschier J. Sequence of the murinefactor VIII cDNA. Genomics 1993;16:374-9.15. Reipert BM, Ahmad RU, Turecek PL, Schwarz HP. Characterizationof antibodies induced by human factor VIIIin a murine knockout model of hemophilia A. ThrombHaemost 2000;84:826-32.16. Sasgary M, Ahmad RU, Schwarz HP, Turecek PL, ReipertBM. Single cell analysis of factor VIII-specific T-cells inhemophilic mice after treatment with human factorVIII. Thromb Haemost 2002;87:266-72.17. Hausl C, Maier E, Schwarz HP, Ahmad RU, Turecek PL,Dorner F, Reipert BM. Long-term persistence of antifactorVIII antibody-secreting cells in hemophilic miceafter treatment with human factor VIII. ThrombHaemost 2002;87:840-5.18. Slifka MK, Antia R, Withmire JK, Ahmed R. Humoralimmunity due to long-lived plasma cells. Immunity1998; 8:363-72.19. Manz RA, Löhning M, Cassese G, Thiel A, Radbruch A.Survival of long-lived plasma cells is independent ofantigen. Intern Immunol 1998;11:1703-11.20. Ochsenbein AF, Pinschewer DD, Sierro S, Horvath E,Hengartner H, Zinkernagel RM. Protective long-termantibody memory by antigen-driven T help-dependentdifferentiation of long-lived memory B-cells to shortlivedplasma cells independent of secondary lymphoidorgans. Proc Am Acad Sci USA 2000;97:13263-8.21. Foy TM, Aruffo A, Bajorath J, Buhlmann JE, Noelle RJ.Immune regulation by CD40 and its ligand GP39. AnnRev Immunol 1996;14:591-617.22. Larsen CP, Alexander DZ, Hollenbaugh D, Elwood ET,Ritchie SC, Aruffo A, et al. CD40-gp39 interactions playa critical role during allograft rejection: suppression ofallograft rejection by blockade of the CD40-gp39 pathway.Transplantation 1996;61:4-9.23. Kirk AD, Burkly LC, Batty DC, Baumgartner RE, BerningJD, Buchanan K. Treatment with humanized monoclonalantibody against CD154 prevents acute renalallograft rejection in nonhuman primates. Nat Med1999;5:686-93.24. Durie FH, Aruffo A, Ledbetter J, Crassi KM, Green WR,Fast LD, et al. Antibody to the ligand of CD40, gp39,blocks the occurrence of the acute and chronic forms ofgraft-vs-host-disease. J Clin Invest 1994;94:1333-8.25. Saito K, Sakurai J, Ohata J, Kohsaka T, Hashimoto H,Okumura K, et al. Involvement of CD40 ligand-CD40and CTLA4-B7 pathways in murine acute graft-versushostdisease induced by allogeneic T cells lacking CD28.J Immunol 1998;160:4225-31.26. Gerritse K, Laman JD, Noeller RJ, Aruffo A, Ledbetter JA,Boersma WJA, et al. CD40-CD40 ligand interactions inexperimental allergic encephalomyelitis and multiplesclerosis. Proc Natl Acad Sci USA 1996;93:2499-504.27. Early GS, Zhao W, Burns CM. Anti-CD40 ligand antibodytreatment prevents the development of lupus-likenephritis in a subset of New Zealand black x NewZealand white mice. Response correlates with theabsence of an anti-antibody response. J Immunol 1996;157:3159-64.28. Taylor PA, Friedman TM, Korngold R, Noelle RJ, BlazarBR. Tolerance induction of alloreactive T cells via ex vivoblockade of the CD40:CD40L costimulatory pathwayresults in the generation of a potent immune regulatorycell. Blood 2002;99:4601-929. Reipert BM, Sasgary M, Ahmad RU, Auer W, TurecekPL, Schwarz HP. Blockade of CD40/CD40 ligand interactionsprevents induction of factor VIII inhibitors inhemophilic mice but does not induce lasting immunetolerance. Thromb Haemost 2001;86:1345-52.30. Qian J, Burkly LC, Smith EP, Ferrant JL, Hoyer LW, ScottDW, et al. Role of CD154 in the secondary immuneresponse: the reduction of pre-existing splenic germinalcenters and anti-factor VIII inhibitor titers. Eur JImmunol 2000;30:2548-54.31. Rossi G, Sarakar J, Scandella D. Long-term induction ofimmune tolerance after blockade of CD40-CD40Linteraction in a mouse model of hemophilia A. Blood2001;97:2750-6.32. Qian J, Collins M, Sharpe AH, Hoyer LW. Preventionand treatment of factor VIII inhibitors in murine hemophiliaA. Blood 2000;95:1324-9.33. Sarkar R, Gao GP, Chirmule N, Tazelaar J, Kazazian HH.Partial correction of the murine hemophilia A with neoantigenicmurine factor VIII. Hum Gene Ther 2000;11:881-94.34. Dazzi F, Rosato A, Tison T, Vianello F, Radossi P, GirolamiA. An animal model to explore the molecular basisof factor VIII (FVIII) inhibitor formation: evidence ofanti-FVIII T-cell response and importance of administrationroute (abstract). Thromb Haemost 1995; 73:1026.35. Jarvis MA, Levin LG, Harrison JA, De Pianto DJ, SuzukiCM, Ziaja CL, et al. Induction of human factor VIIIinhibitors in rats by immunization with human recombinantfactor VIII: a small animal model for humanswith high responder inhibitor phenotype. ThrombHaemost 1996;75:318-25.36. Levin LG, Jarvis M, Powell J, Harrison JA, Reisner HM.Induction of human factor VIII inhibitors in rats 2: finemapping of rat anti-human rFVIII antibodies. ThrombHaemost 1996;76:998-100337. Chao H, Walsh CE. Induction of tolerance to humanfactor VIII in mice. Blood 2001;97:3311-238. Bosma GC, Carroll AM. The SCID mouse mutant: definition,characterization and potential uses. Annu RevImmunol 1991;9:323-50.39. Araki R, Fujimori A, Hamatani K, Mita K, Saito T, MoriM, et al. Nonsense mutation at Tyr-4046 in the DNAdependentprotein kinase catalytic subunit of severecombined immune deficiency mice. Proc Natl Acad SciUSA 1997;94:2438-83.40. Laulan A, Sauger A, Germain C, Montembault AM, SansI, Potentini-Esnault C, et al. Frequency of anti-FVIIIantibodies in humanized SCID mice elicited by recombinantdeleted factor VIII and by plasma derived factorVIII. J Immunol Methods 1997;210:205-14.41. Vanzieleghem B, Gilles JG, Desqueper B, Vermylen J,Saint-Remy JM. Humanized Severe Combined ImmunodeficientMice as a Potential Model for the Study ofTolerance to Factor VIII. Thromb Haemost 2000; 83:833-9.42. Gilles JG, Vanzieleghem B, Saint-Remy JM. Animalmodels to explore mechanisms of tolerance induction toFVIII: SCID mice and SCID-FVIII-KO mice. <strong>Haematologica</strong>2000; 85 Suppl:103-7.haematologica vol. 88(supplement n. 12):september <strong>2003</strong>