2003; baxter - Supplements - Haematologica
2003; baxter - Supplements - Haematologica 2003; baxter - Supplements - Haematologica
36E.P. Mauser-Bunschoten et al.ResultsThe group consisted of 27 patients with severehemophilia A. Patient data are summarized inTables 1 and 2. The median age at inhibitordevelopment was 3 years. Inhibitors developedafter a median of 34 exposures. The median ageat start immune tolerance therapy was 13 years.The total period of follow-up since start ITT was336 years with a median of 13 years per patient.Two patients were lost for follow-up, and 3patients died from Aids.In patient 1-11 factor VIII was continued afteran inhibitor developed, whereas in patient 12 –27 factor VIII was discontinued for at least oneyear.During immune tolerance induction patientswere seen at least every month. We checked thediaries kept by the patients against the amountsof factor VIII supplied to them. Based on thesedata compliance was almost 100%.In 21 patients (78%) success was obtainedwith low dose therapy, in 4 patients dose had tobe adjusted to 75-100 U FVIII/kg bw 3 times aweek before success was seen. They were consideredto be failures. In 2 other patients therapyfailed completely.Success was obtained after 2–28 months. TheKaplan Meier plot of the presence of inhibitor isalmost linear in the first two years of ITT, indicatinga constant chance of disappearance ofinhibitor. Even after 3 years of therapy there is achance the inhibitor will disappear. So farimmune tolerance therapy totally failed in twopatients after 48 and 50 months respectively. Inthese two patients therapy failed even when therapywith high dose factor VIII, intravenous gammaglobulinand cyclophosphamide as describedby Nilsson et al. 10 was given.Logistic regression analyses revealed (Table 3)that there existed a relation between the highestinhibitor level and successful ITT and the timeneeded before success was obtained. Patientswith low inhibitor titers did better. All patientswith an inhibitor level of less than 40 BU/mLwere treated successfully. Patients in whom therapycompletely failed or in whom the dose wasadjusted had inhibitors between 44 BU/mL and753 BU/mL. In patients with maximum inhibitortiter of less than 40 BU/mL success was obtainedwithin 10 months (median 6 months). Inpatients with maximum titers over 40 BU/mLthe median time before success was obtained was18 months , but even after 36 months ITT wassuccessful.Whether therapy was started directly or manyyears after the inhibitor development did notseem to make much difference in this group ofpatients. Treatment with a neutralizing dose atstart of immune tolerance induction did notimprove the results. Furthermore the type ofproduct used to obtain immune tolerance did notaffect the results. Tolerance was also obtainedusing monoclonal purified and recombinantproduct (Table 2).Table 1. Demographic data of patients on immune tolerancetherapy.medianrangeAge at inhibitor development (years) 3 0,5 – 23Number of exposures before inhibitor development 34 8 – 53Age at start ITT (years) 13 1 – 43Period of follow-up (years) 13 4 – 20ComplicationsThe most frequent complication during ITT wasthe occurrence of bleeds. In the beginning bleedswere treated with APCC, and since 1998 withfactor VIIa. When the inhibitor was low and afactor VIII recovery was measured bleeds weretreated with factor VIII. Venous access was problematicin some small children. Therefore in fivepatients Port-a-Cath systems (PAC) wereimplanted at start of ITT when the inhibitor waslow or during ITT under coverage with increaseddose of factor VIII, porcine factor VIII, APCC orcontinuous infusion with factor VIIa. During ITTwe have seen in 3 patient 6 PAC infections. FourPACs were replaced for this reason.Until 1985 seven of 12 patients treated withITT were infected with HIV, 3 of them died fromAids related diseases.In one patient (patient 4) a relapse of theinhibitor was observed one year after he wastolerized. During this period a maximuminhibitor titer of 1 BU/mL was found, withabsence of factor VIII recovery. This patient wastreated with a second course of low dose ITT withgood result. Ten years later he has had no secondrelapse, is on normal dose prophylaxis and hasno spontaneous bleeds.Present statusAt their last visit (Table 4) all patients weretreated with prophylaxis, in most patients bleedsare prevented adequately. In one patient withsevere arthropathy high bleeding frequency isobserved, which probably is caused by the poorphysical condition of this patient. Also in youngpatients with risk behavior a higher bleeding frequencyis seen. However these patient do not sufferfrom spontaneous bleeds.In one patient prophylaxis was stopped duringa period of 10 years without recurrence of theinhibitor. After successful ITT 27 surgical interventionswere performed in 12 patients under factorVIII coverage. Bolus injection as well as continuousinfusion was used with good hemostaticeffect. In none of the patients post operativebleeding occurred.haematologica vol. 88(supplement n. 12):september 2003
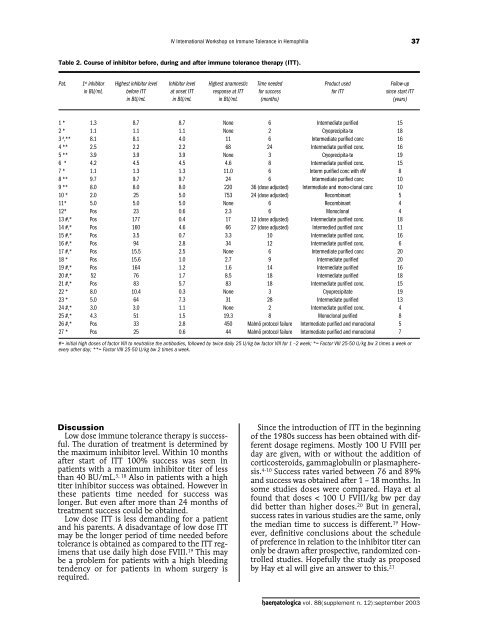
IV International Workshop on Immune Tolerance in Hemophilia37Table 2. Course of inhibitor before, during and after immune tolerance therapy (ITT).Pat. 1 st inhibitor Highest inhibitor level Inhibitor level Highest anamnestic Time needed Product used Follow-upin BU/mL before ITT at onset ITT response at ITT for success for ITT since start ITTin BU/mL in BU/mL in BU/mL (months) (years)1 * 1.3 8.7 8.7 None 6 Intermediate purified 152 * 1.1 1.1 1.1 None 2 Cryoprecipita-te 183 # ,** 8.1 8.1 4.0 11 6 Intermediate purified conc 164 ** 2.5 2.2 2.2 68 24 Intermediate purified conc. 165 ** 3.9 3.9 3.9 None 3 Cryoprecipita-te 196 * 4.2 4.5 4.5 4.6 8 Intermediate purified conc. 157 * 1.1 1.3 1.3 11.0 6 Interm purified conc with vW 88 ** 9.7 9.7 9.7 24 6 Intermediate purified conc 109 ** 8.0 8.0 8.0 220 36 (dose adjusted) Intermediate and mono-clonal conc 1010 * 2.0 25 5.0 753 24 (dose adjusted) Recombinant 511* 5.0 5.0 5.0 None 6 Recombinant 412* Pos 23 0.6 2.3 6 Monoclonal 413 #,* Pos 177 0.4 17 12 (dose adjusted) Intermediate purified conc. 1814 #,* Pos 160 4.6 66 27 (dose adjusted) Intermedied purified conc 1115 #,* Pos 3.5 0.7 3.3 10 Intermediate purified conc. 1616 #,* Pos 94 2.8 34 12 Intermediate purified conc. 617 #,* Pos 15.5 2.5 None 6 Intermediate purified conc 2018 * Pos 15.6 1.0 2.7 9 Intermediate purified 2019 #,* Pos 164 1.2 1.6 14 Intermediate purified 1620 #,* 52 76 1.7 8.5 18 Intermediate purified 1821 #,* Pos 83 5.7 83 18 Intermediate purified conc. 1522 * 8.0 10.4 0.3 None 3 Cryoprecipitate 1923 * 5.0 64 7.3 31 28 Intermediate purified 1324 #,* 3.0 3.0 1.1 None 2 Intermediate purified conc. 425 #,* 4.3 51 1.5 19.3 8 Monoclonal purified 826 #,* Pos 33 2.8 450 Malmö protocol failure Intermediate purified and monoclonal 527 * Pos 25 0.6 44 Malmö protocol failure Intermediate purified and monoclonal 7#= initial high doses of factor VIII to neutralise the antibodies, followed by twice daily 25 U/kg bw factor VIII for 1 –2 week; *= Factor VIII 25-50 U/kg bw 3 times a week orevery other day; **= Factor VIII 25-50 U/kg bw 2 times a week.DiscussionLow dose immune tolerance therapy is successful.The duration of treatment is determined bythe maximum inhibitor level. Within 10 monthsafter start of ITT 100% success was seen inpatients with a maximum inhibitor titer of lessthan 40 BU/mL. 5, 18 Also in patients with a hightiter inhibitor success was obtained. However inthese patients time needed for success waslonger. But even after more than 24 months oftreatment success could be obtained.Low dose ITT is less demanding for a patientand his parents. A disadvantage of low dose ITTmay be the longer period of time needed beforetolerance is obtained as compared to the ITT regimensthat use daily high dose FVIII. 19 This maybe a problem for patients with a high bleedingtendency or for patients in whom surgery isrequired.Since the introduction of ITT in the beginningof the 1980s success has been obtained with differentdosage regimens. Mostly 100 U FVIII perday are given, with or without the addition ofcorticosteroids, gammaglobulin or plasmapheresis.4-10 Success rates varied between 76 and 89%and success was obtained after 1 – 18 months. Insome studies doses were compared. Haya et alfound that doses < 100 U FVIII/kg bw per daydid better than higher doses. 20 But in general,success rates in various studies are the same, onlythe median time to success is different. 19 However,definitive conclusions about the scheduleof preference in relation to the inhibitor titer canonly be drawn after prospective, randomized controlledstudies. Hopefully the study as proposedby Hay et al will give an answer to this. 21haematologica vol. 88(supplement n. 12):september 2003
- Page 2 and 3: haematologicahinformation for autho
- Page 4 and 5: haematologicahImmunobiology of Tole
- Page 6 and 7: [Molecular Genetics]review paperGen
- Page 8 and 9: [Molecular Genetics]review paperGen
- Page 10: 6R.C.R. LjungTable 2. Int database
- Page 17 and 18: IV International Workshop on Immune
- Page 19 and 20: IV International Workshop on Immune
- Page 21 and 22: IV International Workshop on Immune
- Page 23 and 24: IV International Workshop on Immune
- Page 25 and 26: IV International Workshop on Immune
- Page 27 and 28: IV International Workshop on Immune
- Page 29 and 30: IV International Workshop on Immune
- Page 31 and 32: IV International Workshop on Immune
- Page 33 and 34: [Round Table on Immune Tolerance Tr
- Page 35 and 36: IV International Workshop on Immune
- Page 37 and 38: IV International Workshop on Immune
- Page 39: IV International Workshop on Immune
- Page 43 and 44: IV International Workshop on Immune
- Page 45 and 46: IV International Workshop on Immune
- Page 47 and 48: IV International Workshop on Immune
- Page 49 and 50: [Round Table on Immune Tolerance Tr
- Page 51 and 52: IV International Workshop on Immune
- Page 53 and 54: IV International Workshop on Immune
- Page 55 and 56: IV International Workshop on Immune
- Page 57 and 58: IV International Workshop on Immune
- Page 59 and 60: [Immunobiology of Tolerance Inducti
- Page 61 and 62: IV International Workshop on Immune
- Page 63 and 64: IV International Workshop on Immune
- Page 65 and 66: IV International Workshop on Immune
- Page 67 and 68: IV International Workshop on Immune
- Page 69 and 70: IV International Workshop on Immune
- Page 71 and 72: IV International Workshop on Immune
- Page 73 and 74: [New Aspects in Treatment of Hemoph
- Page 75 and 76: [New Aspects in Treatment of Hemoph
- Page 77 and 78: IV International Workshop on Immune
- Page 79 and 80: [New Aspects in Treatment of Hemoph
- Page 81 and 82: IV International Workshop on Immune
- Page 83 and 84: IV International Workshop on Immune
- Page 85 and 86: IV International Workshop on Immune
- Page 87 and 88: IV International Workshop on Immune
- Page 89 and 90: IV International Workshop on Immune
IV International Workshop on Immune Tolerance in Hemophilia37Table 2. Course of inhibitor before, during and after immune tolerance therapy (ITT).Pat. 1 st inhibitor Highest inhibitor level Inhibitor level Highest anamnestic Time needed Product used Follow-upin BU/mL before ITT at onset ITT response at ITT for success for ITT since start ITTin BU/mL in BU/mL in BU/mL (months) (years)1 * 1.3 8.7 8.7 None 6 Intermediate purified 152 * 1.1 1.1 1.1 None 2 Cryoprecipita-te 183 # ,** 8.1 8.1 4.0 11 6 Intermediate purified conc 164 ** 2.5 2.2 2.2 68 24 Intermediate purified conc. 165 ** 3.9 3.9 3.9 None 3 Cryoprecipita-te 196 * 4.2 4.5 4.5 4.6 8 Intermediate purified conc. 157 * 1.1 1.3 1.3 11.0 6 Interm purified conc with vW 88 ** 9.7 9.7 9.7 24 6 Intermediate purified conc 109 ** 8.0 8.0 8.0 220 36 (dose adjusted) Intermediate and mono-clonal conc 1010 * 2.0 25 5.0 753 24 (dose adjusted) Recombinant 511* 5.0 5.0 5.0 None 6 Recombinant 412* Pos 23 0.6 2.3 6 Monoclonal 413 #,* Pos 177 0.4 17 12 (dose adjusted) Intermediate purified conc. 1814 #,* Pos 160 4.6 66 27 (dose adjusted) Intermedied purified conc 1115 #,* Pos 3.5 0.7 3.3 10 Intermediate purified conc. 1616 #,* Pos 94 2.8 34 12 Intermediate purified conc. 617 #,* Pos 15.5 2.5 None 6 Intermediate purified conc 2018 * Pos 15.6 1.0 2.7 9 Intermediate purified 2019 #,* Pos 164 1.2 1.6 14 Intermediate purified 1620 #,* 52 76 1.7 8.5 18 Intermediate purified 1821 #,* Pos 83 5.7 83 18 Intermediate purified conc. 1522 * 8.0 10.4 0.3 None 3 Cryoprecipitate 1923 * 5.0 64 7.3 31 28 Intermediate purified 1324 #,* 3.0 3.0 1.1 None 2 Intermediate purified conc. 425 #,* 4.3 51 1.5 19.3 8 Monoclonal purified 826 #,* Pos 33 2.8 450 Malmö protocol failure Intermediate purified and monoclonal 527 * Pos 25 0.6 44 Malmö protocol failure Intermediate purified and monoclonal 7#= initial high doses of factor VIII to neutralise the antibodies, followed by twice daily 25 U/kg bw factor VIII for 1 –2 week; *= Factor VIII 25-50 U/kg bw 3 times a week orevery other day; **= Factor VIII 25-50 U/kg bw 2 times a week.DiscussionLow dose immune tolerance therapy is successful.The duration of treatment is determined bythe maximum inhibitor level. Within 10 monthsafter start of ITT 100% success was seen inpatients with a maximum inhibitor titer of lessthan 40 BU/mL. 5, 18 Also in patients with a hightiter inhibitor success was obtained. However inthese patients time needed for success waslonger. But even after more than 24 months oftreatment success could be obtained.Low dose ITT is less demanding for a patientand his parents. A disadvantage of low dose ITTmay be the longer period of time needed beforetolerance is obtained as compared to the ITT regimensthat use daily high dose FVIII. 19 This maybe a problem for patients with a high bleedingtendency or for patients in whom surgery isrequired.Since the introduction of ITT in the beginningof the 1980s success has been obtained with differentdosage regimens. Mostly 100 U FVIII perday are given, with or without the addition ofcorticosteroids, gammaglobulin or plasmapheresis.4-10 Success rates varied between 76 and 89%and success was obtained after 1 – 18 months. Insome studies doses were compared. Haya et alfound that doses < 100 U FVIII/kg bw per daydid better than higher doses. 20 But in general,success rates in various studies are the same, onlythe median time to success is different. 19 However,definitive conclusions about the scheduleof preference in relation to the inhibitor titer canonly be drawn after prospective, randomized controlledstudies. Hopefully the study as proposedby Hay et al will give an answer to this. 21haematologica vol. 88(supplement n. 12):september <strong>2003</strong>