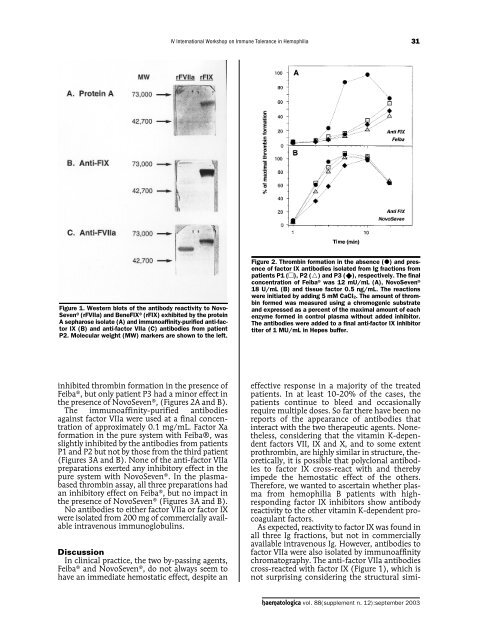
30J. Astermark et al.noglobulins and CNBr-activated sepharose 4 Bwere obtained from Pharmacia & Upjohn.Sepharose 6B coupled with factor IX was kindlyprovided by Dr. Freiburghaus, Excorim. Recombinantfactor X was purchased from EnzymeResearch Laboratories and prothrombin fromICN Biochemicals Inc. Recombinant lipidatedtissue factor was obtained from American DiagnosticaInc. The fibrinogen polymerizationinhibitor H-glycine-proline-arginine-proline-OH(GPRP) was from Calbiochem. The chromogenicsubstrates S-2238 and S-2222 were purchasedfrom Haemochrom Diagnostica AB, factor IXdepletedplasma from Biopool AB and humanserum albumin from Sigma.SamplesImmunoglobulin fractions were isolated fromplasma from three patients with severe hemophiliaB (designated P1, P2 and P3) and a highrespondinginhibitor at the age of 5-6 years usingprotein A sepharose as previously described. 9 Theeluted fractions were dialyzed against 0.008 mMHepes containing 0.139 M NaCl, pH 7.3. TheMalmö inhibitor titer was determined asdescribed elsewhere; 10 one Malmö inhibitor unit(MU) is approximately equal to three Bethesdaunits. Western blotting was performed usingstandard techniques. 11Immunoaffinity purification of factor IX antibodieswas performed using sepharose 6 MB towhich factor IX had been coupled as previouslydescribed. 12 Factor VIIa antibodies were isolatedusing CNBr-activated sepharose 4B to which 3mg of human recombinant factor VIIa was coupledaccording to the instructions of the manufacturer.The factor VIIa gel was pre-eluted with0.1 M Tris-HCl containing 0.5 M NaCl, pH 2.2,and then equilibrated in the same Tris-buffer, pH7.0, before mixing with the antibody fractionovernight at 4°C. The gel was washed with Trisbufferand then eluted using a low pH. The eluatewas immediately neutralized to pH 7.0 andthen dialyzed against the Hepes buffer describedabove.In vitro assaysFormation of thrombin was studied in a plasmasystem at 37°C essentially as described byGallistl et al. 13 In short, we used 2 mM GPRP, 50mL of factor IX-deficient or patient’s plasma andrecombinant relipidated tissue factor at a finalconcentration of 0.5 ng/mL in 0.008 M Hepescontaining 0.139 M NaCl, pH 7.3. The final concentrationof NovoSeven ® ‚ was 18 U/mL correspondingto approximately 50% of the expectedpeak value after bolus injection of 100 µg/kg. 14,15The final concentration of Feiba ® ‚ was titrated togive a similar amount of thrombin formed in theabsence of antibodies as that for NovoSeven ® .The various antibody preparations and/or Hepesbuffer were added to a final volume of 218 µLand the reaction was started by adding 0.1 MCaCl2 to a final concentration of 5 mM. Sampleswere collected at various time points for up to 30min and added to the chromogenic substrate S-2238 (final concentration of 0.22 mM). Afterfive minutes the reaction was terminated byadding 160 µL of 50% HAc and the absorbancewas measured at 405 nm. The amount of thrombinformed at each time point was expressed asa percent of the maximal amount of thrombinformed in the factor IX-deficient control plasma.Each experiment was repeated three times on differentoccasions.Formation of factor Xa was studied in a systemcontaining purified proteins (total volume 1 mL)in 0.008 M Hepes containing 0.139 M NaCl, pH7.3, using factor VIII and factor X at a final concentrationof 1 U/mL and 5 µg/mL, respectively.The concentration of recombinant relipidatedtissue factor was 0.5 ng/mL, NovoSeven ® ‚ 0.25U/mL and Feiba ® ‚ 0.5 U/mL. The various antibodypreparations and/or Hepes buffer wereadded and the reaction mixture incubated for20 min at 37°C. The reaction was started byadding 0.1 M CaCl2 to a final concentration of 5mM and then terminated by retransferring 150µL of the mixture to tubes containing EDTA(final concentration 10 mM) at 5,10, 20, 30, 40and 60 min. S-2222 was used at a concentrationof 0.6 nM and the amount of factor X formedmeasured by the absorbance at 405 nm. Theamount of factor Xa formed at each time pointwas then expressed as a percent of the maximalamount of factor Xa formed in the control mixturewithout antibodies. Each experiment wasrepeated three times on different occasions.ResultsIg fractions from three patients with highrespondingfactor IX inhibitors, i.e. a historicalpeak titer of >10 BU/mL, had been collectedbefore the start of immune tolerance induction(ITI) according to the Malmö model using proteinA sepharose. After dialysis, the inhibitoryeffect of each Ig fraction at a final inhibitor titerof 1 MU was measured in a thrombin assay basedon factor IX-deficient plasma. All three Ig fractionswere found to exert an inhibitory effect onthrombin formation in the presence of Feiba®,whereas no major effect was seen when Novo-Seven®‚ was used as active enzyme (not shown).Each Ig fraction was then subjected to immunoaffinitychromatography on a factor IX- and afactor VIIa sepharose. Antibodies were elutedfrom both gels, and Western blotting confirmedthe reactivity of the eluted antibodies to each factor(Figure 1). None of the purified preparationsexhibited any reactivity to factor VIII, X or IIa.In the pure system with Feiba®, using a factorIX inhibitor titer of 1 MU, factor Xa formationwas inhibited 30% by the anti-factor IX antibodiesfrom patient P2 but was only slightly curtailedby the antibodies from the other two patients(not shown). In the presence of NovoSeven®,no major inhibition was seen. All three isolateshaematologica vol. 88(supplement n. 12):september <strong>2003</strong>
IV International Workshop on Immune Tolerance in Hemophilia31Figure 1. Western blots of the antibody reactivity to Novo-Seven ® (rFVIIa) and BeneFIX ® (rFIX) exhibited by the proteinA sepharose isolate (A) and immunoaffinity-purified anti-factorIX (B) and anti-factor VIIa (C) antibodies from patientP2. Molecular weight (MW) markers are shown to the left.Figure 2. Thrombin formation in the absence () and presenceof factor IX antibodies isolated from Ig fractions frompatients P1 (), P2 () and P3 (), respectively. The finalconcentration of Feiba ® was 12 mU/mL (A), NovoSeven ®18 U/mL (B) and tissue factor 0.5 ng/mL. The reactionswere initiated by adding 5 mM CaCl2. The amount of thrombinformed was measured using a chromogenic substrateand expressed as a percent of the maximal amount of eachenzyme formed in control plasma without added inhibitor.The antibodies were added to a final anti-factor IX inhibitortiter of 1 MU/mL in Hepes buffer.inhibited thrombin formation in the presence ofFeiba ® , but only patient P3 had a minor effect inthe presence of NovoSeven ® ‚ (Figures 2A and B).The immunoaffinity-purified antibodiesagainst factor VIIa were used at a final concentrationof approximately 0.1 mg/mL. Factor Xaformation in the pure system with Feiba®‚ wasslightly inhibited by the antibodies from patientsP1 and P2 but not by those from the third patient(Figures 3A and B). None of the anti-factor VIIapreparations exerted any inhibitory effect in thepure system with NovoSeven ® . In the plasmabasedthrombin assay, all three preparations hadan inhibitory effect on Feiba ® , but no impact inthe presence of NovoSeven ® (Figures 3A and B).No antibodies to either factor VIIa or factor IXwere isolated from 200 mg of commercially availableintravenous immunoglobulins.DiscussionIn clinical practice, the two by-passing agents,Feiba ® and NovoSeven ® , do not always seem tohave an immediate hemostatic effect, despite aneffective response in a majority of the treatedpatients. In at least 10-20% of the cases, thepatients continue to bleed and occasionallyrequire multiple doses. So far there have been noreports of the appearance of antibodies thatinteract with the two therapeutic agents. Nonetheless,considering that the vitamin K-dependentfactors VII, IX and X, and to some extentprothrombin, are highly similar in structure, theoretically,it is possible that polyclonal antibodiesto factor IX cross-react with and therebyimpede the hemostatic effect of the others.Therefore, we wanted to ascertain whether plasmafrom hemophilia B patients with highrespondingfactor IX inhibitors show antibodyreactivity to the other vitamin K-dependent procoagulantfactors.As expected, reactivity to factor IX was found inall three Ig fractions, but not in commerciallyavailable intravenous Ig. However, antibodies tofactor VIIa were also isolated by immunoaffinitychromatography. The anti-factor VIIa antibodiescross-reacted with factor IX (Figure 1), which isnot surprising considering the structural simi-haematologica vol. 88(supplement n. 12):september <strong>2003</strong>