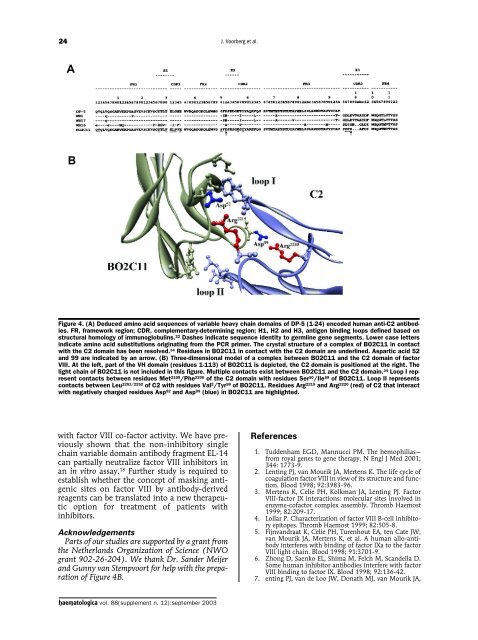
24J. Voorberg et al.ABFigure 4. (A) Deduced amino acid sequences of variable heavy chain domains of DP-5 (1-24) encoded human anti-C2 antibodies.FR, framework region; CDR, complementary-determining region; H1, H2 and H3, antigen binding loops defined based onstructural homology of immunoglobulins. 22 Dashes indicate sequence identity to germline gene segments. Lower case lettersindicate amino acid substitutions originating from the PCR primer. The crystal structure of a complex of BO2C11 in contactwith the C2 domain has been resolved. 34 Residues in BO2C11 in contact with the C2 domain are underlined. Aspartic acid 52and 99 are indicated by an arrow. (B) Three-dimensional model of a complex between BO2C11 and the C2 domain of factorVIII. At the left, part of the VH domain (residues 1-113) of BO2C11 is depicted, the C2 domain is positioned at the right. Thelight chain of BO2C11 is not included in this figure. Multiple contacts exist between BO2C11 and the C2 domain. 34 Loop I representcontacts between residues Met 2199 /Phe 2200 of the C2 domain with residues Ser 50 /Ile 59 of BO2C11. Loop II representscontacts between Leu 2251/2252 of C2 with residues Val 2 /Tyr 98 of BO2C11. Residues Arg 2215 and Arg 2220 (red) of C2 that interactwith negatively charged residues Asp 52 and Asp 99 (blue) in BO2C11 are highlighted.with factor VIII co-factor activity. We have previouslyshown that the non-inhibitory singlechain variable domain antibody fragment EL-14can partially neutralize factor VIII inhibitors inan in vitro assay. 18 Further study is required toestablish whether the concept of masking antigenicsites on factor VIII by antibody-derivedreagents can be translated into a new therapeuticoption for treatment of patients withinhibitors.AcknowledgementsParts of our studies are supported by a grant fromthe Netherlands Organization of Science (NWOgrant 902-26-204). We thank Dr. Sander Meijerand Gunny van Stempvoort for help with the preparationof Figure 4B.References1. Tuddenham EGD, Mannucci PM. The hemophilias—from royal genes to gene therapy. N Engl J Med 2001;344: 1773-9.2. Lenting PJ, van Mourik JA, Mertens K. The life cycle ofcoagulation factor VIII in view of its structure and function.Blood 1998; 92:3983-96.3. Mertens K, Celie PH, Kolkman JA, Lenting PJ. FactorVIII-factor IX interactions: molecular sites involved inenzyme-cofactor complex assembly. Thromb Haemost1999; 82:209-17.4. Lollar P. Characterization of factor VIII B-cell inhibitoryepitopes. Thromb Haemost 1999; 82:505-8.5. Fijnvandraat K, Celie PH, Turenhout EA, ten Cate JW,van Mourik JA, Mertens K, et al. A human allo-antibodyinterferes with binding of factor IXa to the factorVIII light chain. Blood 1998; 91:3701-9.6. Zhong D, Saenko EL, Shima M, Felch M, Scandella D.Some human inhibitor antibodies interfere with factorVIII binding to factor IX. Blood 1998; 92:136-42.7. enting PJ, van de Loo JW, Donath MJ, van Mourik JA,haematologica vol. 88(supplement n. 12):september <strong>2003</strong>
IV International Workshop on Immune Tolerance in Hemophilia 25Mertens K. The sequence Glu1811-Lys1818 of humanblood coagulation factor VIII comprises a binding sitefor activated factor IX. J Biol Chem 1996; 271:1935-40.8. Healey JF, Lubin IM, Nakai H, Saenko EL, Hoyer LW,Scott ME, et al. Residues 484-508 contain a major determinantof the inhibitory epitope in the A2 domain ofhuman factor VIII. J Biol Chem 1995; 270:14505-9.9. Lubin IM, Healey JF, Barrow RT, Scandella D, Lollar P.Analysis of the human factor VIII A2 inhibitor epitopeby alanine scanning mutagenesis. J Biol Chem 1997;272:30191-5.10 Fay PJ, Scandella D. Human inhibitor antibodies specificfor the A2 domain disrupt the interaction between thesubunit and factor IXa. J Biol Chem 1999; 274:29826-30.11 Scandella D, Gilbert GE, Shima M, Nakai H, EaglesonC, Felch M, et al. Some factor VIII inhibitor antibodiesrecognize a common epitope corresponding to C2domain amino acids 2248 through 2312, which overlapa phospholipid- binding site. Blood 1995; 86:1811-9.12 Healey JF, Barrow RT, Tamim HM, Lubin IM, Shima M,Scandella D, et al. Residues Glu2181-Val2243 containa major determinant of the inhibitory epitope in the C2domain of human factor VIII. Blood 1998; 92:3701-9.13. Arai M, Scandella D, Hoyer LW. Molecular basis of factorVIII inhibition by human antibodies. Antibodies thatbind to the factor VIII light chain prevent the interactionof factor VIII with phospholipid. J Clin Invest 1989;83: 1978-84.14 Matsuda K, Ishii K, Bourvagnet P, Kuma KI, HayashidaH, Miyata T, et al. The complete nucleotide sequence ofthe human immunoglobulin heavy chain variableregion locus. J Exp Med 1998; 188:2151-62.15 Tomlinson IM, Williams SC, Ignatovitch O, Corbett SJ,Winter G. V-BASE sequence directory. MRC Centre forProtein Engineering, Cambridge, UK.16 Cook GP, Tomlinson IM. The human immunoglobulinrepertoire. Immunol Today 1995; 16:237-42.17 Rajewski K. Clonal selection and learning in the antibodysystem. Nature 1996; 381:751-8.18 Voorberg J, van den Brink EN. Phage display, a tool toexplore the diversity of inhibitors to blood coagulationfactor VIII. Semin Thromb Haemost 2000; 26:143-50.19 Schier R, Bye J, Apell G, McCall A, Adams GP,Malmqvist M, et al. Isolation of high-affinity monomerichuman anti-c-erB-2 single chain Fv using affinitydrivenselection. J Mol Biol 1996; 255:28-43.20 Griffin HM, Ouwehand WH. A human monoclonalantibody specific for the leucine-33 (P1A1, HPA-1a)form of platelet glycoprotein IIIa from a V gene phagedisplay library. Blood 1995; 86:4430-6.21 de Wildt RM, Hoet RM, van Venrooij WJ, TomlinsonIM, Winter G. Analysis of heavy and light chain pairingsindicates that receptor editing shapes the human antibodyrepertoire. J Mol Biol 1999; 285:895-901.22 Chothia C, Lesk AM, Gherardi E, Tomlinson IM, WalterG, Marks JD, et al. Structural repertoire of thehuman VH segments. J Mol Biol 1992; 227:799-817.23 Van den Brink EN, Turenhout EA, Davies J, BovenschenN, Fijnvandraat K, Ouwehand WH, et al. Human antibodieswith specificity for the C2 domain are derivedfrom VH1 germline genes. Blood 2000; 95:558-63.24 Van den Brink EN, Turenhout EA, Bank C, FijnvandraatK, Peters M, Voorberg J. Molecular analysis of humananti-factor VIII antibodies by V gene phage dsiplay identifiesa new epitope in the acidic region following the A2domain. Blood 2000b; 96:540-5.25 van den Brink EN, Turenhout EA, Bovenschen N, HeijnenBG, Mertens K, Peters M, et al. Multiple VH genesare used to assemble human antibodies directed towardthe A3-C1 domains of factor VIII. Blood 2001; 97:966-72.26 van den Brink EN, Bril WS, Turenhout EA, Zuurveld M,Bovenschen N, Peters M, et al. Two classes of germlinegenes both derived from the V(H)1 family direct theformation of human antibodies that recognize distinctantigenic sites in the C2 domain of factor VIII. Blood2002; 99:2828-34.27. Bril WS, Turenhout EA, Kaijen PH, van den Brink EN,Koopman MM, Peters M, et al. Analysis of factor VIIIinhibitors in a haemophilia A patient with an Arg593-→Cys mutation using phage display. Thromb Haemost2001; 86:247a[abstract].28 Prescott R, Nakai H, Saenko EL, Scharrer I, Nilsson IM,Humphries JE, et al. Recombinate and Kogenate studygroups. The inhibitor antibody responnse is more complexin hemophilia A patients than in most nonhemophiliacswith factor VIII autoantibodies. Blood1997; 89:3663-71.29. Arai M, Imai T, Yuguchi M, Nakashima, Kukutake K.Cloning and characterization of single chain Fv of antifactorVIII antibody derived from a hemophilia A patientwith factor VIII inhibitor. Thromb Haemost 1999; 82:238a[abstract].30 Jacquemin MG, Desqueper BG, Benhida A, van der ElstL, Hoylaerts MF, Bakkus M, et al. Mechanism and kineticsof factor VIII inactivation: study with an IgG4 monoclonalantibody derived from a hemophilia A patientwith an inhibitor. Blood 1998; 92:496-506.31 Kabat EA, Wu TT, Perry HM, Gottesman KS, Foeller C.Sequences of immunological interest. 1991; US Departmentof Health and Human Services: 5 th edition. Bethesda,MD, USA.32 Tramontano A, Chothia C, Lesk AM. Framework residue71 is a major determinant of the position abd conformationof the second hypervariable region in the VHdomains of immunoglobulins. J Mol Biol 1990; 215:175-82.33 Pratt KP, Shen BW, Takeshima K, Davie EW, FujikawaK , Stoddard BL. Structure of the C2 domain of humanfactor VIII at 1.5 Å resolution. Nature 1999; 402:439-42.34 Spiegel PC, Jacquemin M, Saint Remy JM, Stoddard BL,Pratt KP. Structure of a factor VIII C2 domain-immunoglobulinG4k Fab complex: identification of aninhibitor antibody epitope on the surface of factor VIII.Blood 2001; 98:13-9.35. Barrow RT, Healey JF, Jacquemin MG, Saint-Remy JM,Lollar P. Antigenicity of putative phospholipid membranebinding residues in factor VIII. Blood 2001;97:169-74.36 Gilbert GE, Kaufman RJ, Arena AA, Miao H, Pipe SW.Four hydrophobic amino acids of the factor VIII C2domain are constituents of both the membrane-bindingand von Willebrand factor-binding motifs. J Biol Chem2002; 277:6374-81.haematologica vol. 88(supplement n. 12):september <strong>2003</strong>