ATOM MODEL PROJECT PURPOSE: To create a 3-D ... - InforMNs
ATOM MODEL PROJECT PURPOSE: To create a 3-D ... - InforMNs
ATOM MODEL PROJECT PURPOSE: To create a 3-D ... - InforMNs
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>ATOM</strong> <strong>MODEL</strong> <strong>PROJECT</strong><br />
(by F. <strong>To</strong>dd 2004)<br />
<strong>PURPOSE</strong>: <strong>To</strong> <strong>create</strong> a 3-D model of an atom.<br />
PROCEDURE:<br />
1. Your teacher will assign you an atom for your model.<br />
The name of my atom is:______________________<br />
2. You will do some research and record the following information about your atom:<br />
A. Atomic number:_________________<br />
B. Atomic mass:___________________<br />
C. Mass number:___________________<br />
D. Number of Protons:______________<br />
E. Number of Neutrons:_____________<br />
F. Number of Electrons:_____________<br />
G. Arrangement of Electrons: K___ L___ M___<br />
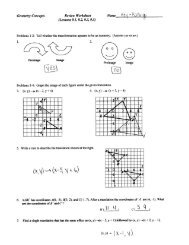
F. Make a 2-D sketch of your atom in the space below.<br />
3. Use the information that you acquired from step #2 to construct a 3-D model of<br />
your atom. Your model must show the following:<br />
A. Be 3-Dimensional with the protons, neutrons, and electrons<br />
represented in their correct locations and by their correct respective sizes.<br />
B. Have a key attached to it that states the atoms<br />
-Name<br />
-Number of protons<br />
-Number of neutrons<br />
-Number of electrons<br />
-Electron shell arrangement of the electrons<br />
-Your name and period<br />
C. Contain a string and paper clip so that you teacher can hang it in the room<br />
or have a stand so that your model can be displayed on the counter.<br />
GRADING: Will be based on how well you carry out the above procedures and neatness.<br />
EXTRA CREDIT: 3 extra points will be awarded to any atom that can fit inside a 5 x 5 x 5 inch box.
<strong>ATOM</strong> <strong>MODEL</strong> <strong>PROJECT</strong> GRADING RUBRIC<br />
NAME:_______________________________________________________________________________________ PER:____<br />
Atom model<br />
Correct number of protons & placement of protons in the model: ____________ (2)<br />
Correct number of neutrons & placement of neutrons in the model: ____________ (2)<br />
Correct number of electrons & placement of electrons in the model: ____________ (2)<br />
Respective sizes of protons, neutrons, and electrons is correct: ____________ (3)<br />
(i.e. protons and neutrons are the same size electrons are smaller)<br />
Key<br />
Contains the students name and period: ____________ (2)<br />
Contains the atoms name ____________ (2)<br />
Correctly identifies the protons, neutrons, and electrons ____________ (3)<br />
Correctly tells how many electrons are in the K, L, M, shells ____________ (4)<br />
Overall Construction & Neatness<br />
Did not do Poor Average Above Outstanding ____________ (10)<br />
Average<br />
(0) (4) (6) (8) (10)<br />
Extra Credit ____________ (0)<br />
<strong>To</strong>tal Points ____________ (30)<br />
---------------------------------------------------------------------------------------------------------------------------------------------------------------------------------<br />
<strong>ATOM</strong> <strong>MODEL</strong> <strong>PROJECT</strong> GRADING RUBRIC<br />
NAME:_______________________________________________________________________________________ PER:____<br />
Atom model<br />
Correct number of protons & placement of protons in the model: ____________ (2)<br />
Correct number of neutrons & placement of neutrons in the model: ____________ (2)<br />
Correct number of electrons & placement of electrons in the model: ____________ (2)<br />
Respective sizes of protons, neutrons, and electrons is correct: ____________ (3)<br />
(i.e. protons and neutrons are the same size electrons are smaller)<br />
Key<br />
Contains the students name and period: ____________ (2)<br />
Contains the atoms name ____________ (2)<br />
Correctly identifies the protons, neutrons, and electrons ____________ (3)<br />
Correctly tells how many electrons are in the K, L, M, shells ____________ (4)<br />
Overall Construction & Neatness<br />
Did not do Poor Average Above Outstanding ____________ (10)<br />
Average<br />
(0) (4) (6) (8) (10)<br />
Extra Credit ____________ (0)<br />
<strong>To</strong>tal Points ____________ (30)