Partial Nephrectomy: Indications and relevant investigations
Partial Nephrectomy: Indications and relevant investigations Partial Nephrectomy: Indications and relevant investigations
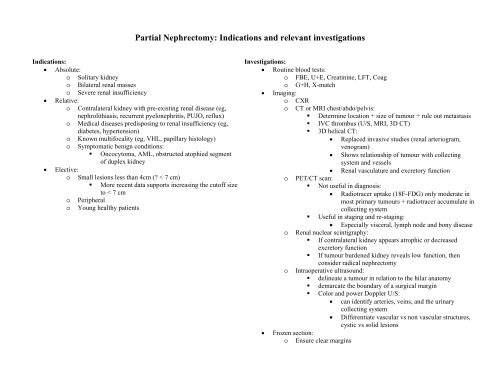
Partial Nephrectomy: Indications and relevant investigations Indications: • Absolute: o Solitary kidney o Bilateral renal masses o Severe renal insufficiency • Relative: o Contralateral kidney with pre-existing renal disease (eg, nephrolithiasis, recurrent pyelonephritis, PUJO, reflux) o Medical diseases predisposing to renal insufficiency (eg, diabetes, hypertension) o Known multifocality (eg, VHL, papillary histology) o Symptomatic benign conditions: • Oncocytoma, AML, obstructed atophied segment of duplex kidney • Elective: o Small lesions less than 4cm (? < 7 cm) • More recent data supports increasing the cutoff size to < 7 cm o Peripheral o Young healthy patients Investigations: • Routine blood tests: o FBE, U+E, Creatinine, LFT, Coag o G+H, X-match • Imaging: o CXR o CT or MRI chest/abdo/pelvis: • Determine location + size of tumour + rule out metastasis • IVC thrombus (U/S, MRI, 3D CT) • 3D helical CT: • Replaced invasive studies (renal arteriogram, venogram) • Shows relationship of tumour with collecting system and vessels • Renal vasculature and excretory function o PET/CT scan: • Not useful in diagnosis: • Radiotracer uptake (18F-FDG) only moderate in most primary tumours + radiotracer accumulate in collecting system • Useful in staging and re-staging: • Especially visceral, lymph node and bony disease o Renal nuclear scintigraphy: • If contralateral kidney appears atrophic or decreased excretory function • If tumour burdened kidney reveals low function, then consider radical nephrectomy o Intraoperative ultrasound: • delineate a tumour in relation to the hilar anatomy • demarcate the boundary of a surgical margin • Color and power Doppler U/S: • can identify arteries, veins, and the urinary collecting system • Differentiate vascular vs non vascular structures, cystic vs solid lesions • Frozen section: o Ensure clear margins
- Page 2 and 3: Management of Metastatic Renal Cell
- Page 4 and 5: Role of Tyrosine Kinase Inhibitor (
- Page 6 and 7: Management of Caval Tumour T3b and
- Page 8 and 9: Lymphadenopathy in Renal Tumours Di
- Page 10 and 11: Management of Renal Cell recurrence
- Page 13: Case Presentation and Discussion Em
<strong>Partial</strong> <strong>Nephrectomy</strong>: <strong>Indications</strong> <strong>and</strong> <strong>relevant</strong> <strong>investigations</strong><br />
<strong>Indications</strong>:<br />
• Absolute:<br />
o Solitary kidney<br />
o Bilateral renal masses<br />
o Severe renal insufficiency<br />
• Relative:<br />
o Contralateral kidney with pre-existing renal disease (eg,<br />
nephrolithiasis, recurrent pyelonephritis, PUJO, reflux)<br />
o Medical diseases predisposing to renal insufficiency (eg,<br />
diabetes, hypertension)<br />
o Known multifocality (eg, VHL, papillary histology)<br />
o Symptomatic benign conditions:<br />
• Oncocytoma, AML, obstructed atophied segment<br />
of duplex kidney<br />
• Elective:<br />
o Small lesions less than 4cm (? < 7 cm)<br />
• More recent data supports increasing the cutoff size<br />
to < 7 cm<br />
o Peripheral<br />
o Young healthy patients<br />
Investigations:<br />
• Routine blood tests:<br />
o FBE, U+E, Creatinine, LFT, Coag<br />
o G+H, X-match<br />
• Imaging:<br />
o CXR<br />
o CT or MRI chest/abdo/pelvis:<br />
• Determine location + size of tumour + rule out metastasis<br />
• IVC thrombus (U/S, MRI, 3D CT)<br />
• 3D helical CT:<br />
• Replaced invasive studies (renal arteriogram,<br />
venogram)<br />
• Shows relationship of tumour with collecting<br />
system <strong>and</strong> vessels<br />
• Renal vasculature <strong>and</strong> excretory function<br />
o PET/CT scan:<br />
• Not useful in diagnosis:<br />
• Radiotracer uptake (18F-FDG) only moderate in<br />
most primary tumours + radiotracer accumulate in<br />
collecting system<br />
• Useful in staging <strong>and</strong> re-staging:<br />
• Especially visceral, lymph node <strong>and</strong> bony disease<br />
o Renal nuclear scintigraphy:<br />
• If contralateral kidney appears atrophic or decreased<br />
excretory function<br />
• If tumour burdened kidney reveals low function, then<br />
consider radical nephrectomy<br />
o Intraoperative ultrasound:<br />
• delineate a tumour in relation to the hilar anatomy<br />
• demarcate the boundary of a surgical margin<br />
• Color <strong>and</strong> power Doppler U/S:<br />
• can identify arteries, veins, <strong>and</strong> the urinary<br />
collecting system<br />
• Differentiate vascular vs non vascular structures,<br />
cystic vs solid lesions<br />
• Frozen section:<br />
o Ensure clear margins
Management of<br />
Metastatic Renal Cell<br />
Carcinoma -2007<br />
Sunny Lee WA<br />
• RCC<br />
Most lethal cancer in Urology<br />
•<br />
Cancer specific mortality >40% 1<br />
<br />
20-30% present with Mets 2<br />
20-40% undergoing<br />
nephrectomy for localised<br />
RCC with develop Mets<br />
Overall median survival for<br />
M1
• Resection of mets can lead to<br />
long term survival<br />
• Metachronous mets do better<br />
than synchronous mets<br />
•<br />
resection of isolated Lung Mets<br />
especially favourable 1<br />
<br />
<br />
Better outcome if complete<br />
metastasectomy <strong>and</strong>
Role of Tyrosine Kinase<br />
Inhibitor (2)<br />
• Motzer 1 et al,<br />
<br />
<br />
<br />
<br />
750 pts, no previous systemic Tx,<br />
no brain mets, no high CVS risk,<br />
ECOG 0/1, clear cell histo, 90%<br />
prev CRN<br />
R<strong>and</strong>omised: Oral Sunitinib 50mg<br />
od vs SC IFNα 3x/wk<br />
Continue until progression<br />
Results (for Sunitinib grp):<br />
• 6 mth median survival advantage<br />
(11 vs 5)<br />
• Higher response rate (31% vs 6%)<br />
• Similar S/E profile (more diarrhoea<br />
in Sunitinib)<br />
• Better quality of life<br />
Identifying good risk patients<br />
through better underst<strong>and</strong>ing<br />
of tumour biology<br />
• Tumour vaccine therapy<br />
• Defining role of CRN<br />
Especially with<br />
antiangiogenic therapy<br />
• Adjuvant therapy with TKI<br />
ECOG Trial E2805<br />
SORCE Trial<br />
Role of Tyrosine Kinase<br />
Inhibitor (2)<br />
• Escudier 1 et al<br />
903 pts, progression post<br />
systemic Tx, ECOG 0/1,<br />
intermediate/low risk grp<br />
Oral sorafenib 400mg bd vs<br />
placebo<br />
Median survival benefit 2.7<br />
mths (5.5 vs 2.8)<br />
• S/E of TKI<br />
Diarrhoea, rash, fatigue,<br />
h<strong>and</strong>-foot skin reactions,<br />
hypertension, cardiac<br />
ischaemia<br />
Management Flow<br />
Chart<br />
The future<br />
• Refining patient selection<br />
for immunotherapy
ACTIVE SURVEILLANCE & INNOVATIVE TREATMENT OF RENAL CELL CANCER.<br />
D.Gyomber<br />
Increased imaging has resulted in frequent detection of small renal masses. (SRM) Most in patients older than 70 yrs with<br />
competing medical co-morbidities. Hollingsworth et al evaluating SEER data determined the relative benefit of renal cancer<br />
surgery declines with decreasing tumour stage <strong>and</strong> increasing age. Increased treatment of SRM has not decreased mortality,<br />
therefore not all SRM require treatment, analogous to prostate cancer.<br />
Natural History. SRM is < 3cm. Larger SRM are more likely to be malignant, but the ratio of malignant to benign is<br />
approximately the same regardless of size. i.e. 80-90% of SRM are malignant. Schlomer et al J Urol 2006 reviewed 345 renal<br />
lesions 2-2-9cm were benign <strong>and</strong> malignant 22.9 <strong>and</strong> 77.1% of the time respectively <strong>and</strong> those 3-3.9cm 17.5% <strong>and</strong> 82.5%<br />
Tumour size <strong>and</strong> pathological findings in 287 SRM
Management of Caval Tumour<br />
T3b <strong>and</strong> T3c RCC is associated with a particular biological propensity for vascular<br />
invasion, with up to 10% of patients having associated venous tumour thrombus<br />
involving the renal vein or inferior vena cava <strong>and</strong> 1% having a tumour thrombus that<br />
extends into the right atrium.<br />
Staging techniques-<br />
• Ultrasound (Pre <strong>and</strong> Intra op)<br />
• CT scan<br />
• MRI<br />
• Cavography<br />
• Trans-oesophagial echo<br />
To identify involvement of renal vein <strong>and</strong> inferior vena cava<br />
To determine the cephalic extent of the tumour<br />
For preoperative planning<br />
Requirement of Hepatic mobilization<br />
Requirement of cardiac bypass<br />
Assessment of transmural invasion<br />
Surgical management<br />
Preoperative cardiovascular <strong>and</strong> pulmonary evaluation <strong>and</strong> therapy<br />
Early ligation of the renal artery to decrease the chance of venous bleeding<br />
?Need for Preoperative arterial embolization<br />
Thrombus extension at <strong>and</strong> above the level of the hepatic veins poses a particular<br />
challenge because of limited access <strong>and</strong> difficulty in vascular control<br />
Team Approach<br />
The only curative approach to renal cell carcinoma is surgery. An aggressive<br />
approach is warranted when tumor involves the renal vein <strong>and</strong> inferior vena cava.<br />
Surgical strategy depends on the level of the inferior vena caval thrombus. Patients<br />
with extension of the thrombus above the diaphragm are a greater technical<br />
challenge. Hypothermic circulatory arrest should be considered when treating vena<br />
caval-atrial tumor thrombus.<br />
References<br />
1. The Mayo Clinic experience with surgical management, complications <strong>and</strong> outcome for<br />
patients with renal cell carcinoma <strong>and</strong> venous tumour thrombus. 2004 BJU International<br />
2. American College of Radiology criteria 2007<br />
3. Surgical techniques for treating a Renal neoplasm invading the inferior vena cava JUROL Feb<br />
2003<br />
4. Multidetector computed tomography vs magnetic resonance imaging for defining the upper<br />
limit of tumour thrombus in renal cell carcinoma: a study <strong>and</strong> review. Nathan Lawrentschuk<br />
2005 BJU International
Management of small renal tumours in the elderly patient.<br />
Evolution of management of small tumours driven by new technologies <strong>and</strong> recognition that total<br />
nephrectomies predispose patients to chronic renal failure <strong>and</strong> it’s associated morbidity.<br />
• RCC changing epidemiology:<br />
Increase in incidence, especially in incidental finding of small (
Lymphadenopathy in Renal Tumours<br />
Discussion<br />
Robson (1969)<br />
• Radical nephrectomy with lymphadenectomy<br />
• Para-aortic <strong>and</strong> paracaval lymphnodes from bifurcation of the aorta to crus of<br />
diaphragm<br />
Arguments for<br />
• Improve accuracy of staging<br />
• Decrease recurrence rates<br />
• Improve survival<br />
Arguments against<br />
• Equal frequency of blood <strong>and</strong> lymph spread.<br />
• many patients get mets without LN involvment<br />
• Lymphatic drainage of kidney is variable<br />
• Only a small subset of patients will benefit (2-3%)<br />
• Micrometastatic disease<br />
Evidence<br />
1. EORTC study 30881<br />
• No difference in rate of 5yr progression or survival in patients with nonmetastatic<br />
disease with or without LND<br />
2. Pantuck et al. J Urol 2003<br />
• Lymphadenectomy had a role in patients for cytoreductive nephrectomy with<br />
grossly enlarged lymph nodes.<br />
3. Simmons et al. Urol 2007<br />
• Laparoscopic LND is feasible in select population.<br />
Minimal impact on perioperative morbidity<br />
Increased operating time 30min (experts, learning curve)<br />
Unable to assess if lap LND is as efficacious as open (would require RCT but still<br />
difficult to detect a difference)<br />
Case selection: plane around ipsilateral great vessel.
Bleeding During <strong>Nephrectomy</strong> – Dr Conrad V Bishop<br />
• 56yo man smoker<br />
• Works in IT industry<br />
• PHx : - obesity<br />
- type 2 DM - hypertension<br />
- gout<br />
• Perforated appendix 30 years ago<br />
• Advised right radical nephrectomy<br />
• Offered laparoscopic nephrectomy<br />
•<br />
• H<strong>and</strong> assisted right radical nephrectomy<br />
• H<strong>and</strong> port via old appendicectomy scar<br />
•<br />
• Right colon <strong>and</strong> duodenum mobilised<br />
• Kidney elevated by assistant, vessels dissected with hook diathermy<br />
•<br />
• When suddenly…………. Major bleeding<br />
• Calmly alert anaesthetist - fluid resuscitation<br />
- aramine - get blood products<br />
- baseline bloods (FBE, coags inc, fibrinogen)<br />
•<br />
• Get help<br />
• Request open setup, 2 suckers<br />
• Right supracostal incision (12th rib)<br />
•<br />
• Most haemorrhage is venous from avulsion injury<br />
• Avulsion of lumbar vein – posterior to renal vein<br />
– posterior to IVC<br />
• Avulsion of gonadal vein – from IVC on right<br />
– from left renal vein<br />
• Avulsion of adrenal vein<br />
• Direct injury to IVC<br />
•<br />
5mm tear in IVC<br />
Anterior at level of renal vein<br />
Likely hook diathermy injury<br />
Repaired with 5.0 prolene
Management of Renal Cell recurrence<br />
Ahmed Al-Sameraaii<br />
History<br />
Examination<br />
Laboratory testing<br />
Imaging<br />
Surgical <strong>and</strong> non Surgical ablative procedures<br />
Palliative<br />
*********************************************************<br />
APPROACH<br />
Review type of Surgery :after RN or NSS<br />
Determine site of recurrence :Local (LR) or systemic (SR)<br />
Determine magnitude of recurrence<br />
Local recurrence (LR) of RCC after radical nephrectomy is an uncommon event,<br />
occurring in approximately 2% to 4% of cases.<br />
LR after RN is rare in patients with low-stage pT1-2 N0 M0 RCC.<br />
Only about 40% of local recurrences are isolated; the majority of patients with<br />
local recurrence had original T3 or above <strong>and</strong> also have systemic recurrence<br />
(SR), <strong>and</strong> a thorough metastatic evaluation should be pursued.<br />
Personal History<br />
Smoking ( increase risk of recurrence 2 fold),Healthy life style!<br />
Rarely other associated tumours cerebelar hemangioblastomas <strong>and</strong> retinal<br />
angiomata (VHL disease)<br />
S & S of flank pain, hematuria, wt loss, bone pain , fever cough , hemoptysis<br />
Exam :mass , sudden left varicocele , focal neurological signs, Lymph node dis<br />
Lab Microscopic hematuria, Anaemia, LFT,, increased Hb, Calcium<br />
Imaging<br />
CT C+/-<br />
Most sensitive <strong>and</strong> cost effective single imaging modality, 85% of such<br />
masses are RCC<br />
MRI<br />
CNS imaging is essential in advanced disease<br />
USS<br />
to evaluate cystic mass<br />
Risk factors include 1) increasing T stage 2) Pathological<br />
staging ,unfavourable pathology <strong>and</strong> N positive. 3)Familial ( VHL)<br />
Local recurrence in the remnant kidney after nephron-sparing surgery for RCC<br />
is low <strong>and</strong> has been reported in 1.4% to 10% of patients, <strong>and</strong> the main risk<br />
factor is advanced T stage.<br />
Treatment<br />
Treatment of Local Recurrence<br />
Treatment of LocoRegiona diseasel<br />
Systemic<br />
Remember that most recurrences are distant from the tumour bed.<br />
Probably a result of unrecognized tumour multicentricity or de novo<br />
occurrence<br />
__________________________________
Isolated local recurrence after Radical <strong>Nephrectomy</strong> (RN)>>>>Surgical<br />
resection if feasible or EBRT *<br />
Surgery can provide long-term cancer-free status for about 35%<br />
Often a formidable task because the natural tissue barriers are no longer present<br />
<strong>and</strong> invasion of contiguous organs is not uncommon. En bloc resection of<br />
adjacent organs is often required, <strong>and</strong> the risk of morbidity can be substantial<br />
Radiation therapy may be of value for palliation of symptomatic local recurrence<br />
in patients who are not operative c<strong>and</strong>idates.<br />
Options for treatment<br />
Recurrence after PN > repeat PN **<br />
or Completion nephrectomy<br />
or RFA*>repeat RFA or surgery<br />
or Cryo>repeat or surgery<br />
Recurrence after RN>Remove local recurrence***<br />
>Adjuvant<br />
>Palliative<br />
>resect the solitary met
Case Presentation <strong>and</strong> Discussion<br />
Embolisation of Renal Cell Carcinoma<br />
Case Report<br />
Mr. PD<br />
65 year old male<br />
Vague abdominal pain for 6 months<br />
GP – ordered CT scan<br />
Referred to urology clinic with CT<br />
Plan<br />
Operative – open anterior left nephrectomy<br />
Pre-operative embolisation considered<br />
Discussion<br />
Embolisation has role in embolisation of<br />
renal cell carcinoma for palliation of<br />
haematuria <strong>and</strong> for pre-operative renal<br />
artery control<br />
Occasional used for AMLs <strong>and</strong> patients<br />
unfit for surgery<br />
Embolisation can be selective (entire<br />
kidney) or nonselective (segmental)<br />
Three embolising modalities: coils,<br />
alcohol, microspheres<br />
Post-infarction syndrome lasts 1-3 days –<br />
includes loin pain, fevers, nausea<br />
Large left cyst <strong>and</strong> solid cystic renal mass<br />
Close association to spleen <strong>and</strong> artery<br />
Case Conclusion<br />
Proceeded to surgery without embolisation<br />
Surgery complicated by splenic bleeding<br />
<strong>and</strong> high drain output (?pancreatitis)<br />
Histopathology demonstrated<br />
rhabdomyosarcoma<br />
Embolisation Conclusion<br />
Role of embolisation declining <strong>and</strong> remains<br />
controversial<br />
Studies into embolisation small<br />
Palliative embolisation for haematuria<br />
main current application<br />
Selective embolisation available<br />
Large left renal vein<br />
Short left renal artery posterior to vein<br />
References<br />
Munro et al 2003. The role of transarterial<br />
embolisation in the treatment of renal cell<br />
cancer. BJU – 92:240-244<br />
Maxwell et al 2007. Renal artery<br />
embolisation in the palliative treatment of<br />
renal carcinoma. British Journal of<br />
Radiology - 80:96-102
METASTATIC RCC – CASE PRESENTATIONS<br />
CASE 1<br />
79yo man<br />
• 4 month wt loss (4-5kg) <strong>and</strong> lethargy<br />
• Fe deficiency anaemia<br />
• Mild SOBOE<br />
• Ex-smoker<br />
• Asbestosis<br />
– interstitial lung disease<br />
– extensive pleural plaques<br />
– FEV1 50% predicted<br />
CT abdo – large R upper pole mass abutting liver ?metatasis or direct invasion<br />
CT chest – 3-4x pulmonary metastasis (biopsied)<br />
Confirmed met RCC<br />
Hb 108 ↓, Plt 407↑, LFT normal, Corr Ca 2.75↑<br />
Due to poor performance status, age <strong>and</strong> comorbidities – unlikely to benefit from debulking<br />
(cytoreductive) nephrectomy which is likely to require an open approach +/- liver resection.<br />
Role of nephrectomy unclear with new systemic therapies targeting angiogenesis pathways<br />
Medical oncology referral for consideration for targeted systemic therapy (Sutent)<br />
CASE 2<br />
46yo man<br />
• R radical nephrectomy 1997 (G3T1) - Mackay<br />
• Rectus muscle metastasis 2002<br />
– solitary 2cm nodule<br />
– excision biopsied<br />
– clear cell renal cell carcinoma<br />
– margins clear<br />
• No immunotherapy<br />
• Annual surveillance USS/CT scans<br />
• USS showed solid left mid pole kidney mass (3.6cm) confirmed on CT Sept 2007<br />
• Primary or secondary?<br />
• Asymptomatic<br />
• Hb 123, Creat 86 (eGFR>60), LDH 180, mildly elevated LFT (hep C)<br />
• Staging CT chest showed solitary left hila mass 3.6cm<br />
• Bone scan NAD<br />
• PET scan showed small area of uptake left gluteus muscle (2cm nodule)<br />
• USS guided biopsies confirmed metastatic RCC<br />
Issues<br />
1. Role of metastasectomy <strong>and</strong> benefits uncertain (multiple lesions)<br />
2. Solitary kidney - difficult partial nephrectomy <strong>and</strong> may render dialysis dependent<br />
3. Pulmonary metastasis - hilar lesion ?resectability<br />
4. Gluteus metastasis – prognostic significance<br />
Currently being considered for systemic targeted therapy (tyrosine kinase)