Tese_Tânia Vieira.pdf - Ubi Thesis
Tese_Tânia Vieira.pdf - Ubi Thesis
Tese_Tânia Vieira.pdf - Ubi Thesis
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chapter III – Results and Discussion<br />
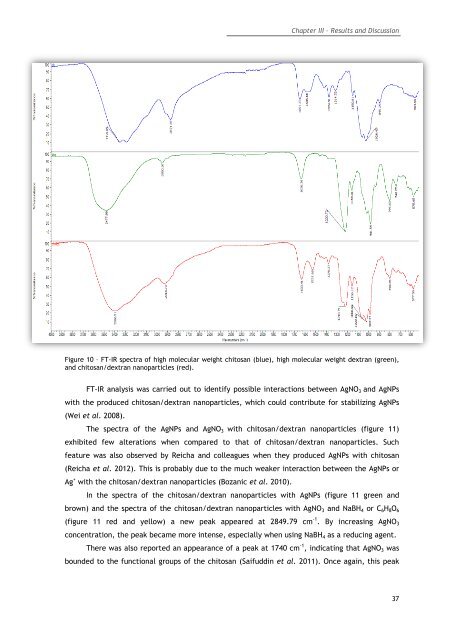
Figure 10 – FT-IR spectra of high molecular weight chitosan (blue), high molecular weight dextran (green),<br />
and chitosan/dextran nanoparticles (red).<br />
FT-IR analysis was carried out to identify possible interactions between AgNO 3 and AgNPs<br />
with the produced chitosan/dextran nanoparticles, which could contribute for stabilizing AgNPs<br />
(Wei et al. 2008).<br />
The spectra of the AgNPs and AgNO 3 with chitosan/dextran nanoparticles (figure 11)<br />
exhibited few alterations when compared to that of chitosan/dextran nanoparticles. Such<br />
feature was also observed by Reicha and colleagues when they produced AgNPs with chitosan<br />
(Reicha et al. 2012). This is probably due to the much weaker interaction between the AgNPs or<br />
Ag + with the chitosan/dextran nanoparticles (Bozanic et al. 2010).<br />
In the spectra of the chitosan/dextran nanoparticles with AgNPs (figure 11 green and<br />
brown) and the spectra of the chitosan/dextran nanoparticles with AgNO 3 and NaBH 4 or C 6 H 8 O 6<br />
(figure 11 red and yellow) a new peak appeared at 2849.79 cm -1 . By increasing AgNO 3<br />
concentration, the peak became more intense, especially when using NaBH 4 as a reducing agent.<br />
There was also reported an appearance of a peak at 1740 cm -1 , indicating that AgNO 3 was<br />
bounded to the functional groups of the chitosan (Saifuddin et al. 2011). Once again, this peak<br />
37