Affinity Chromatography - Department of Molecular and Cellular ...
Affinity Chromatography - Department of Molecular and Cellular ...
Affinity Chromatography - Department of Molecular and Cellular ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
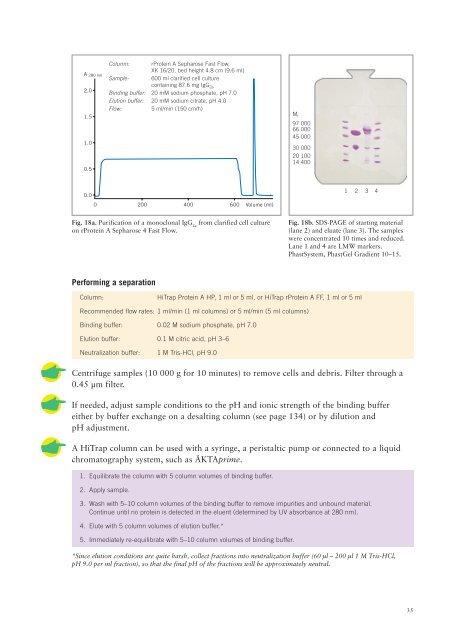
A 280 nm<br />
2.0<br />
1.5<br />
1.0<br />
0.5<br />
Column: rProtein A Sepharose Fast Flow,<br />
XK 16/20, bed height 4.8 cm (9.6 ml)<br />
Sample: 600 ml clarified cell culture<br />
containing 87.6 mg IgG 2a<br />
Binding buffer: 20 mM sodium phosphate, pH 7.0<br />
Elution buffer: 20 mM sodium citrate, pH 4.0<br />
Flow:<br />
5 ml/min (150 cm/h)<br />
M r<br />
97 000<br />
66 000<br />
45 000<br />
30 000<br />
20 100<br />
14 400<br />
0.0<br />
0 200 400 600 Volume (ml)<br />
1 2 3 4<br />
Fig. 18a. Purification <strong>of</strong> a monoclonal IgG 2a<br />
from clarified cell culture<br />
on rProtein A Sepharose 4 Fast Flow.<br />
Fig. 18b. SDS-PAGE <strong>of</strong> starting material<br />
(lane 2) <strong>and</strong> eluate (lane 3). The samples<br />
were concentrated 10 times <strong>and</strong> reduced.<br />
Lane 1 <strong>and</strong> 4 are LMW markers.<br />
PhastSystem, PhastGel Gradient 10–15.<br />
Performing a separation<br />
Column:<br />
HiTrap Protein A HP, 1 ml or 5 ml, or HiTrap rProtein A FF, 1 ml or 5 ml<br />
Recommended flow rates: 1 ml/min (1 ml columns) or 5 ml/min (5 ml columns)<br />
Binding buffer: 0.02 M sodium phosphate, pH 7.0<br />
Elution buffer: 0.1 M citric acid, pH 3–6<br />
Neutralization buffer: 1 M Tris-HCl, pH 9.0<br />
Centrifuge samples (10 000 g for 10 minutes) to remove cells <strong>and</strong> debris. Filter through a<br />
0.45 µm filter.<br />
If needed, adjust sample conditions to the pH <strong>and</strong> ionic strength <strong>of</strong> the binding buffer<br />
either by buffer exchange on a desalting column (see page 134) or by dilution <strong>and</strong><br />
pH adjustment.<br />
A HiTrap column can be used with a syringe, a peristaltic pump or connected to a liquid<br />
chromatography system, such as ÄKTAprime.<br />
1. Equilibrate the column with 5 column volumes <strong>of</strong> binding buffer.<br />
2. Apply sample.<br />
3. Wash with 5–10 column volumes <strong>of</strong> the binding buffer to remove impurities <strong>and</strong> unbound material.<br />
Continue until no protein is detected in the eluent (determined by UV absorbance at 280 nm).<br />
4. Elute with 5 column volumes <strong>of</strong> elution buffer.*<br />
5. Immediately re-equilibrate with 5–10 column volumes <strong>of</strong> binding buffer.<br />
*Since elution conditions are quite harsh, collect fractions into neutralization buffer (60 µl – 200 µl 1 M Tris-HCl,<br />
pH 9.0 per ml fraction), so that the final pH <strong>of</strong> the fractions will be approximately neutral.<br />
35