Influence of the Processes Parameters on the Properties of The ...
Influence of the Processes Parameters on the Properties of The ...
Influence of the Processes Parameters on the Properties of The ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Chapter 2.<br />
<str<strong>on</strong>g>Processes</str<strong>on</strong>g> to Manufacture Foams and to Functi<strong>on</strong>alize <str<strong>on</strong>g>the</str<strong>on</strong>g> Surface<br />
Super critical fluid Technologies, although envir<strong>on</strong>mentally friendly and suitable for mass<br />
producti<strong>on</strong>, requires specially designed equipment and is more expensive. In <str<strong>on</strong>g>the</str<strong>on</strong>g> early days, supercritical<br />
fluids were mainly used in extracti<strong>on</strong> and chromatography applicati<strong>on</strong>s [Smith, 1999; Dean, 1998;<br />
Vandenburg et al., 1997; McNally, 1995; Brunner, 1994; Hedrick et al., 1992]. A well-known example <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
supercritical fluid extracti<strong>on</strong> is caffeine extracti<strong>on</strong> from tea and c<str<strong>on</strong>g>of</str<strong>on</strong>g>fee [McHugh and Kruk<strong>on</strong>is, 1994].<br />
Supercritical chromatography was frequently used to separate polar compounds [Berger, 1997; Cantrell and<br />
Blackwell, 1997]. Nowadays, an increasing interest is being shown in supercritical fluid applicati<strong>on</strong>s for<br />
reacti<strong>on</strong>, catalysis, polymerizati<strong>on</strong>, polymer processing, and polymer modificati<strong>on</strong> [Eckert et al., 1996]. SCF<br />
technologies are now emerging as an alternative to c<strong>on</strong>venti<strong>on</strong>al materials processing methods in <str<strong>on</strong>g>the</str<strong>on</strong>g> area <str<strong>on</strong>g>of</str<strong>on</strong>g><br />
tissue engineering [Duarte et al., 2009a; Duarte et al., 2009b]. ScCO 2 processing may be used to form<br />
foamed scaffolds in which <str<strong>on</strong>g>the</str<strong>on</strong>g> escape <str<strong>on</strong>g>of</str<strong>on</strong>g> CO 2 from a plasticized polymer melt generates gas bubbles that<br />
shape <str<strong>on</strong>g>the</str<strong>on</strong>g> developing pores.<br />
3.3 Scaffolds Prepared by Phase Inversi<strong>on</strong> using scCO 2 as Anti-solvent<br />
Phase inversi<strong>on</strong> using supercritical CO 2 as antis-olvent is analogous to traditi<strong>on</strong>al phase inversi<strong>on</strong><br />
with immersi<strong>on</strong> precipitati<strong>on</strong>. This technique c<strong>on</strong>sists <str<strong>on</strong>g>of</str<strong>on</strong>g> immersing a thin film <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> polymer soluti<strong>on</strong> in a<br />
bath c<strong>on</strong>taining a n<strong>on</strong>-solvent (with respect to <str<strong>on</strong>g>the</str<strong>on</strong>g> polymer). <strong>The</strong> properties <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> final porous structure are<br />
mainly c<strong>on</strong>trolled by <str<strong>on</strong>g>the</str<strong>on</strong>g> precipitati<strong>on</strong> temperature, <str<strong>on</strong>g>the</str<strong>on</strong>g> strength <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> n<strong>on</strong>-solvent bath and <str<strong>on</strong>g>the</str<strong>on</strong>g> compositi<strong>on</strong><br />
<str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> casting soluti<strong>on</strong>. <strong>The</strong> use <str<strong>on</strong>g>of</str<strong>on</strong>g> a supercritical fluid as an antisolvent allows for <str<strong>on</strong>g>the</str<strong>on</strong>g> tuning <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g><br />
antisolvent strength simply by regulating <str<strong>on</strong>g>the</str<strong>on</strong>g> pressure. As a c<strong>on</strong>sequence, <str<strong>on</strong>g>the</str<strong>on</strong>g> pressure is an additi<strong>on</strong>al<br />
parameter for tailoring <str<strong>on</strong>g>the</str<strong>on</strong>g> final structure [Tsivintzelis et al., 2007a].<br />
<strong>The</strong> use <str<strong>on</strong>g>of</str<strong>on</strong>g> CO 2 as an antisolvent for <str<strong>on</strong>g>the</str<strong>on</strong>g> producti<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> porous structures with polymers has not<br />
been thoroughly investigated. Since <str<strong>on</strong>g>the</str<strong>on</strong>g> majority <str<strong>on</strong>g>of</str<strong>on</strong>g> foaming methods applied in <str<strong>on</strong>g>the</str<strong>on</strong>g> semicrystalline<br />
polymers involve <str<strong>on</strong>g>the</str<strong>on</strong>g> use <str<strong>on</strong>g>of</str<strong>on</strong>g> organic solvents, <str<strong>on</strong>g>the</str<strong>on</strong>g>re is an important advantage <str<strong>on</strong>g>of</str<strong>on</strong>g> using phase inversi<strong>on</strong> in <str<strong>on</strong>g>the</str<strong>on</strong>g><br />
presence <str<strong>on</strong>g>of</str<strong>on</strong>g> supercritical CO 2 . With this technique, it is possible to dry <str<strong>on</strong>g>the</str<strong>on</strong>g> final polymer structure simply by<br />
flashing <str<strong>on</strong>g>the</str<strong>on</strong>g> pressure vessel with fresh CO 2 . Thus, <str<strong>on</strong>g>the</str<strong>on</strong>g>re is no need for additi<strong>on</strong>al post-treatment in order to<br />
remove <str<strong>on</strong>g>the</str<strong>on</strong>g> residual organic solvent [Tsivintzelis et al., 2007a]. Dichloromethane can be selected as solvent<br />
since it is completely miscible with CO 2 at pressures higher than 95 bars and temperatures up to 55 o C<br />
[Tsivintzelis et al., 2004]. Additi<strong>on</strong>ally, <str<strong>on</strong>g>the</str<strong>on</strong>g> solubility <str<strong>on</strong>g>of</str<strong>on</strong>g> P L LA in CO 2 at <str<strong>on</strong>g>the</str<strong>on</strong>g>se c<strong>on</strong>diti<strong>on</strong>s is negligible,<br />
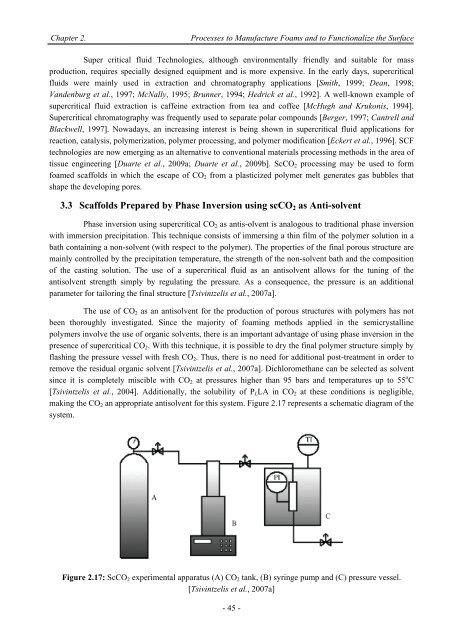
making <str<strong>on</strong>g>the</str<strong>on</strong>g> CO 2 an appropriate antisolvent for this system. Figure 2.17 represents a schematic diagram <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g><br />
system.<br />
Figure 2.17: ScCO 2 experimental apparatus (A) CO 2 tank, (B) syringe pump and (C) pressure vessel.<br />
[Tsivintzelis et al., 2007a]<br />
- 45 -