Influence of the Processes Parameters on the Properties of The ...
Influence of the Processes Parameters on the Properties of The ...
Influence of the Processes Parameters on the Properties of The ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chapter 3.<br />
Analytical Methods and Designs <str<strong>on</strong>g>of</str<strong>on</strong>g> Experiments<br />
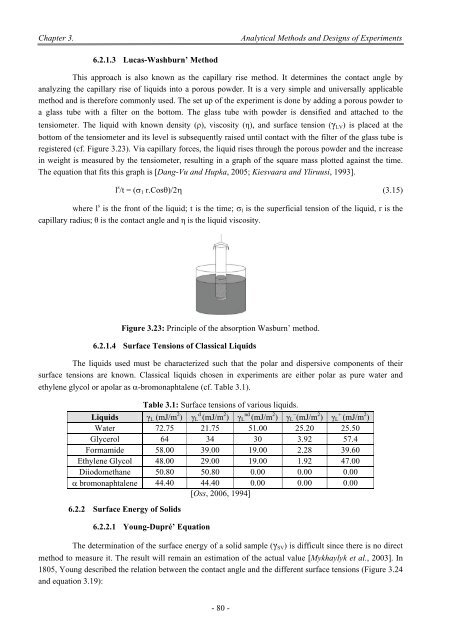
6.2.1.3 Lucas-Washburn’ Method<br />
This approach is also known as <str<strong>on</strong>g>the</str<strong>on</strong>g> capillary rise method. It determines <str<strong>on</strong>g>the</str<strong>on</strong>g> c<strong>on</strong>tact angle by<br />
analyzing <str<strong>on</strong>g>the</str<strong>on</strong>g> capillary rise <str<strong>on</strong>g>of</str<strong>on</strong>g> liquids into a porous powder. It is a very simple and universally applicable<br />
method and is <str<strong>on</strong>g>the</str<strong>on</strong>g>refore comm<strong>on</strong>ly used. <strong>The</strong> set up <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> experiment is d<strong>on</strong>e by adding a porous powder to<br />
a glass tube with a filter <strong>on</strong> <str<strong>on</strong>g>the</str<strong>on</strong>g> bottom. <strong>The</strong> glass tube with powder is densified and attached to <str<strong>on</strong>g>the</str<strong>on</strong>g><br />
tensiometer. <strong>The</strong> liquid with known density (ρ), viscosity (η), and surface tensi<strong>on</strong> (γ LV ) is placed at <str<strong>on</strong>g>the</str<strong>on</strong>g><br />
bottom <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> tensiometer and its level is subsequently raised until c<strong>on</strong>tact with <str<strong>on</strong>g>the</str<strong>on</strong>g> filter <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> glass tube is<br />
registered (cf. Figure 3.23). Via capillary forces, <str<strong>on</strong>g>the</str<strong>on</strong>g> liquid rises through <str<strong>on</strong>g>the</str<strong>on</strong>g> porous powder and <str<strong>on</strong>g>the</str<strong>on</strong>g> increase<br />
in weight is measured by <str<strong>on</strong>g>the</str<strong>on</strong>g> tensiometer, resulting in a graph <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> square mass plotted against <str<strong>on</strong>g>the</str<strong>on</strong>g> time.<br />
<strong>The</strong> equati<strong>on</strong> that fits this graph is [Dang-Vu and Hupka, 2005; Kiesvaara and Yliruusi, 1993].<br />
l s /t = ( r.Cos (3.15)<br />
where l s is <str<strong>on</strong>g>the</str<strong>on</strong>g> fr<strong>on</strong>t <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> liquid; t is <str<strong>on</strong>g>the</str<strong>on</strong>g> time; l is <str<strong>on</strong>g>the</str<strong>on</strong>g> superficial tensi<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> liquid, r is <str<strong>on</strong>g>the</str<strong>on</strong>g><br />
capillary radius; θ is <str<strong>on</strong>g>the</str<strong>on</strong>g> c<strong>on</strong>tact angle and η is <str<strong>on</strong>g>the</str<strong>on</strong>g> liquid viscosity.<br />
Figure 3.23: Principle <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> absorpti<strong>on</strong> Wasburn’ method.<br />
6.2.1.4 Surface Tensi<strong>on</strong>s <str<strong>on</strong>g>of</str<strong>on</strong>g> Classical Liquids<br />
<strong>The</strong> liquids used must be characterized such that <str<strong>on</strong>g>the</str<strong>on</strong>g> polar and dispersive comp<strong>on</strong>ents <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g>ir<br />
surface tensi<strong>on</strong>s are known. Classical liquids chosen in experiments are ei<str<strong>on</strong>g>the</str<strong>on</strong>g>r polar as pure water and<br />
ethylene glycol or apolar as -brom<strong>on</strong>aphtalene (cf. Table 3.1).<br />
Table 3.1: Surface tensi<strong>on</strong>s <str<strong>on</strong>g>of</str<strong>on</strong>g> various liquids.<br />
Liquids γ L (mJ/m 2 ) γ d L (mJ/m 2 ) γ nd L (mJ/m 2 ) γ − L (mJ/m 2 ) γ + L (mJ/m 2 )<br />
Water 72.75 21.75 51.00 25.20 25.50<br />
Glycerol 64 34 30 3.92 57.4<br />
Formamide 58.00 39.00 19.00 2.28 39.60<br />
Ethylene Glycol 48.00 29.00 19.00 1.92 47.00<br />
Diiodomethane 50.80 50.80 0.00 0.00 0.00<br />
brom<strong>on</strong>aphtalene 44.40 44.40 0.00 0.00 0.00<br />
[Oss, 2006, 1994]<br />
6.2.2 Surface Energy <str<strong>on</strong>g>of</str<strong>on</strong>g> Solids<br />
6.2.2.1 Young-Dupré’ Equati<strong>on</strong><br />
<strong>The</strong> determinati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> surface energy <str<strong>on</strong>g>of</str<strong>on</strong>g> a solid sample (γ SV ) is difficult since <str<strong>on</strong>g>the</str<strong>on</strong>g>re is no direct<br />
method to measure it. <strong>The</strong> result will remain an estimati<strong>on</strong> <str<strong>on</strong>g>of</str<strong>on</strong>g> <str<strong>on</strong>g>the</str<strong>on</strong>g> actual value [Mykhaylyk et al., 2003]. In<br />
1805, Young described <str<strong>on</strong>g>the</str<strong>on</strong>g> relati<strong>on</strong> between <str<strong>on</strong>g>the</str<strong>on</strong>g> c<strong>on</strong>tact angle and <str<strong>on</strong>g>the</str<strong>on</strong>g> different surface tensi<strong>on</strong>s (Figure 3.24<br />
and equati<strong>on</strong> 3.19):<br />
- 80 -