Quick Frozen Fish Fillet
Quick Frozen Fish Fillet
Quick Frozen Fish Fillet
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
11<br />
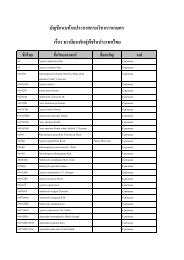
TAS 7014-2005<br />
ANNEX B-3<br />
Sampling and Decision Criteria<br />
In compliance with the provisions of the relevant laws and regulations in particular the latest<br />
version.<br />
1. Defectives<br />
The total number of "defects" shall not exceed the acceptance numbers (c) of the appropriate<br />
sampling plan.<br />
Sampling Plan<br />
Lot size Sampling size (n) Acceptance (c)<br />
200 or less 3 0<br />
201 – 800 6 1<br />
801 – 1,600 13 2<br />
1,601 – 2,400 21 3<br />
2,401 – 3,600 29 4<br />
Over 3,600 38 5<br />
A sample unit may be a primary container at least a 1 kg portion of the sample unit.<br />
2. Microorganism and Food additives 1<br />
2.1 Random sampling shall be carried out with 5 samples per lot.<br />
2.2 The remaining samples microorganism shall be used in the determination of food<br />
additives. For the determination of food additives, take the remaining samples of from each<br />
box or parcel at least 50 g. Thoroughly grind the sample and consequently use for the<br />
determination of food additives.<br />
2.3 All results shall conform to Section 7.2 and Section 4 of this standard.<br />
3. Veterinary drug residues 2<br />
3.1 Random sampling shall be carried out by collect 12 subsamples . Minimum subsample size is<br />
1 kg.<br />
3.2 Minimum quantity required for laboratory sample is 1000 g<br />
3.3 All results shall conform to Section 6 of this standard.<br />
Source:<br />
1. Thai Industrial Standard Institute, B.E.2529 (1986). Industrial Standards:<br />
<strong>Quick</strong> <strong>Frozen</strong> <strong>Fish</strong> <strong>Fillet</strong>, TIS 616-2529 (1986), Ministry of Industry, Bangkok.<br />
2. Codex Alimentarius Volume 3. Residues of Veterinary Drugs in Foods. Codex<br />
Guidelines for the Establishment of a Regulatory Programme for Control of<br />
Veterinary Drug Residues in Foods (CAC/GL 16-1993) Joint FAO/WHO Food<br />
Standard Programme, FAO, Rome.