Org. Chem II Experiment 9 Synthesis of Luminol - OChemOnline
Org. Chem II Experiment 9 Synthesis of Luminol - OChemOnline
Org. Chem II Experiment 9 Synthesis of Luminol - OChemOnline
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Org</strong>. <strong>Chem</strong> <strong>II</strong> <strong>Experiment</strong> 9 <strong>Synthesis</strong> <strong>of</strong> <strong>Luminol</strong> 6<br />
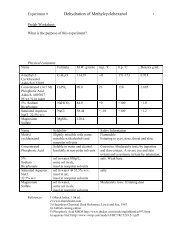
Name Formula M.W. m.p. o C b.p. o C Density g/mL<br />
g/mole<br />
3-nitrophthalic acid C 8 H 5 NO 6 211.13 213-216<br />
hydrazine N 2 H 4 32.05 2.0 113.5 1.0036<br />
triethylene glycol C 6 H 14 O 4 150.17 -7.2 285 1.1274<br />
10% sodium hydroxide NaOH 40.0 1M sol<br />
~4<br />
1M sol.<br />
~102<br />
1 M sol.<br />
~1.0<br />
sodium dithionite Na 2 O 4 S 2 174.11<br />
(Sodium Hydrosulfite)<br />
glacial acetic acid C 2 H 4 O 2 60.05 16.7 118 1.049<br />
potassium hydroxide KOH 56.11 About 360<br />
Dimethyl<br />
C 2 H 6 OS 78.14 18.45 189 1.100<br />
Sulfoxide (DMSO)<br />
luminol C 8 H 7 N 3 O 2 177.16 319-320<br />
Name Solubility Safety Information<br />
3-nitrophthalic soluble in water,<br />
Warning: irritating to skin, eyes and<br />
acid<br />
slightly soluble in alcohol<br />
mucous membranes. Avoid skin contact<br />
hydrazine<br />
triethylene glycol<br />
10% sodium<br />
hydroxide<br />
sodium dithionite<br />
Sodium<br />
Hydrosulfite<br />
glacial acetic acid<br />
potassium<br />
hydroxide<br />
Dimethyl<br />
sulfoxide<br />
<strong>Luminol</strong><br />
References:<br />
insoluble in nonpolar solvents<br />
Miscible with water<br />
Miscible with alcohol<br />
Slightly miscible in nonpolar<br />
Miscible with water<br />
Miscible with alcohol<br />
Slightly miscible in nonpolar<br />
soluble in water,<br />
soluble in alc,<br />
insoluble in nonpolar solvents<br />
Very soluble in water<br />
Slightly soluble in alcohol<br />
Insoluble in nonpolar solvents<br />
Miscible with water,<br />
Miscible with alcohol,<br />
some miscible with nonpolar solvents.<br />
Soluble in water<br />
Slighly soluble in alcohol<br />
Insoluble in nonpolar sovents<br />
Miscible with water,<br />
Miscible with alcohol,<br />
some miscible with nonpolar solvents.<br />
soluble in water,<br />
slightly soluble in alcohol<br />
insoluble in nonpolar solvents<br />
1) Merck Index, 11th ed.<br />
2) www.chemfinder.com<br />
3) Hazardous <strong>Chem</strong>ical Desk Reference, Lewis and Sax, 1987<br />
4) Aldrich catalog online<br />
Warning: Corrosive.<br />
Warning: Irritant Warning Explosive<br />
Danger: Carcinogen.<br />
Poison by intravenous route.<br />
Mild toxicity.<br />
Combustible.<br />
Warning: Corrosive<br />
Warning: irritating to skin, eyes and<br />
mucous membranes. Avoid skin contact<br />
Caution: Flammable Solid<br />
Corrosive!<br />
Moderately toxic by various routes.<br />
Moderate fire and explosion hazard.<br />
Danger: corrosive, Danger: caustic,<br />
hygroscopic, Poison,<br />
Irritant and corrosive to membranes.<br />
Skin and Eye Irritant.<br />
Teratogen.<br />
Moderately toxic.<br />
Warning: irritating to skin, eyes and<br />
mucous membranes. Avoid skin contact