download report - Sapienza
download report - Sapienza
download report - Sapienza
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Scientific Report 2007-2009<br />
Condensed matter physics and biophysics<br />
C14. Statistical Biophysics<br />
Cellular metabolism is to a large extent inaccessible<br />
to experiments. The best experimental technique available<br />
to date to probe intracellular reactions rely on C13-<br />
based flux analysis: basically, a population of cells (eg<br />
bacteria) is prepared to grow in a medium with a labeled<br />
carbon source (eg glucose). After a transient, the marker<br />
reaches a steady distribution over cells, and the mass-tocharge<br />
ratio distribution in certain target compounds (eg<br />
alanine) can be detected via mass or NMR spectroscopy.<br />
From this, reaction fluxes can be inferred. In a model<br />
system like the bacterium E.Coli, this allows to infer<br />
the values of a few tens of fluxes (all from the major<br />
carbohydrate-processing pathways) out of the roughly<br />
1100 forming its metabolism. Much less is known about<br />
eukaryotic cells, and only a handful of data cover human<br />
cells (including the highly important red blood cells).<br />
The inherent difficulty of gathering experimental evidence<br />
makes theoretical approaches a necessary instrument<br />
to reconstruct the global organization of fluxes at<br />
the cellular level, both to predict responses to environmental<br />
perturbations, drugs, or gene knockouts, and to<br />
infer the critical epistatic interactions between metabolic<br />
genes. Several methods are currently available to compute<br />
reaction fluxes from the known stoichiometry, one<br />
prominent example being flux balance analysis. The pillars<br />
on which all of them rest are the assumptions that<br />
(a) metabolite concentrations and reaction fluxes are at<br />
a steady state, and (b) the cell’s overall activity aims<br />
at maximizing the production and/or the consumption<br />
of a given set of metabolites. The latter condition is<br />
typically expressed via a linear optimization problem.<br />
Biomass maximization and ATP maximization, glucose<br />
consumption minimization or total flux minimization are<br />
all examples of this kind of approach.<br />
Some experimental evidence indeed has shown that E.<br />
Coli under evolutionary pressure evolves towards states<br />
of maximal biomass production in nutrient rich environments.<br />
These models are able to reproduce the limited<br />
empirical evidence with a varying degree of success. In<br />
particular, if wild type cells in certain environments may<br />
be reasonably well described by a biomass optimization<br />
principle, after a knockout they are best described by<br />
a principle of minimal flux adjustment with respect to<br />
the wild type. Over the last few years, however, several<br />
limitations of the existing theories have emerged, most<br />
strikingly in the proliferation of objective functions that<br />
are needed to describe different cells, environments and<br />
cell mutants. By standard approaches it is not possible<br />
to predict the biomass composition given the cell and<br />
its environment: rather, the detailed biomass composition<br />
is a key input of the models. A further drawback<br />
of available theories is that by linear optimization they<br />
systematically reduce the space of feasible flux states to<br />
a single point (the optimum). Most of the biological<br />
features requiring high flexibility, like metabolic pathways<br />
coregulation or flux reorganization after knockouts<br />
or environmental changes, are unlikely to be captured by<br />
a simple optimization scheme.<br />
Understanding the cell’s response to perturbations at<br />
the metabolic level requires a deeper analysis of the existing<br />
data and theories, besides new heuristics to explore<br />
different directions. Our group has tackled such<br />
problem within Von Neumann’s maximal producibility<br />
framework. The scientific novelty of our research lies essentially<br />
in the possibility to identify essential reactions<br />
(or genes) as dynamically stiff ones, thus linking directly<br />
to the genetic level. Results obtained so far reproduce<br />
the experimental evidence and allow to infer (rather than<br />
assume) the biomass composition. Many interesting extensions<br />
are currently being analyzed.<br />
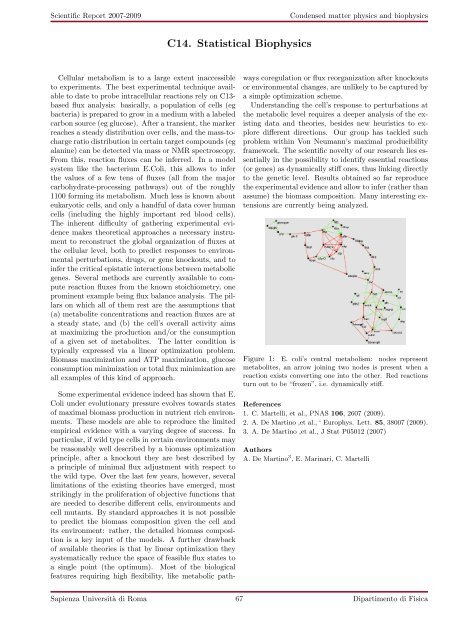
Figure 1: E. coli’s central metabolism: nodes represent<br />
metabolites, an arrow joining two nodes is present when a<br />
reaction exists converting one into the other. Red reactions<br />
turn out to be “frozen”, i.e. dynamically stiff.<br />
References<br />
1. C. Martelli, et al., PNAS 106, 2607 (2009).<br />
2. A. De Martino ,et al., ‘ Europhys. Lett. 85, 38007 (2009).<br />
3. A. De Martino ,et al., J Stat P05012 (2007)<br />
Authors<br />
A. De Martino 3 , E. Marinari, C. Martelli<br />
<strong>Sapienza</strong> Università di Roma 67 Dipartimento di Fisica