Printable PDF Version - Gore Medical
Printable PDF Version - Gore Medical Printable PDF Version - Gore Medical
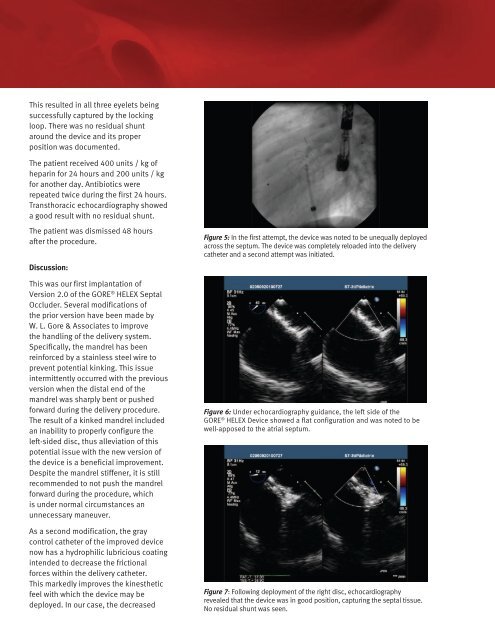
This resulted in all three eyelets being successfully captured by the locking loop. There was no residual shunt around the device and its proper position was documented. The patient received 400 units / kg of heparin for 24 hours and 200 units / kg for another day. Antibiotics were repeated twice during the first 24 hours. Transthoracic echocardiography showed a good result with no residual shunt. The patient was dismissed 48 hours after the procedure. Discussion: This was our first implantation of Version 2.0 of the GORE ® HELEX Septal Occluder. Several modifications of the prior version have been made by W. L. Gore & Associates to improve the handling of the delivery system. Specifically, the mandrel has been reinforced by a stainless steel wire to prevent potential kinking. This issue intermittently occurred with the previous version when the distal end of the mandrel was sharply bent or pushed forward during the delivery procedure. The result of a kinked mandrel included an inability to properly configure the left-sided disc, thus alleviation of this potential issue with the new version of the device is a beneficial improvement. Despite the mandrel stiffener, it is still recommended to not push the mandrel forward during the procedure, which is under normal circumstances an unnecessary maneuver. As a second modification, the gray control catheter of the improved device now has a hydrophilic lubricious coating intended to decrease the frictional forces within the delivery catheter. This markedly improves the kinesthetic feel with which the device may be deployed. In our case, the decreased Figure 5: In the first attempt, the device was noted to be unequally deployed across the septum. The device was completely reloaded into the delivery catheter and a second attempt was initiated. Figure 6: Under echocardiography guidance, the left side of the GORE ® HELEX Device showed a flat configuration and was noted to be well-apposed to the atrial septum. Figure 7: Following deployment of the right disc, echocardiography revealed that the device was in good position, capturing the septal tissue. No residual shunt was seen.
friction contributed to a non optimal configuration of the left disc. However, this was easily corrected by controlling both the gray control catheter and the green delivery catheter together during the deployment of the left atrial disc. Figure 8: Fluoroscopy demonstrated normal configuration of the GORE ® HELEX Septal Occluder. The third modification to the device includes the substitution of the existing retrieval cord with a stronger cord. This component, as with the previous version of the device, enables retrieval of the device after full deployment and release from the delivery system. The modified retrieval cord allows for higher tensile forces to be applied before a potential cord break occurs. In our experience this might become important if the device got entangled within intra-atrial structures, like the Eustachian valve. In this case, higher forces may be needed to overcome the resistance and pull the device down to the inferior vena cava for retrieval into the delivery catheter. Conclusion: Figure 9: Following lock release, the locking loop was noted to have captured all three eyelets. Figure 10: Echocardiography revealed the final position of the device following removal of the retrieval cord. The right and left discs were in good apposition with the atrial septum, and no residual shunt was noted. The modifications embodied in Version 2.0 of the GORE ® HELEX Septal Occluder offer a series of noted benefits without significantly altering the implantation procedure associated with deployment of the device. These modifications enhance the usability of the device by reducing the rate for potential delivery system issues. Overall the new delivery system supports straight forward implantation procedures with a good final result more so than the previous versions of the GORE ® HELEX Septal Occluder. Please refer to GORE ® HELEX Septal Occluder Instructions for Use at goremedical.com for a complete description of all indications, contraindications, warnings, precautions and adverse events.
- Page 1 and 2: Closing Rem a rk s In This I S S U
- Page 3: was exchanged for a 10 Fr introduce
- Page 7 and 8: I N T H E N E W S Gore Launches Imp
This resulted in all three eyelets being<br />
successfully captured by the locking<br />
loop. There was no residual shunt<br />
around the device and its proper<br />
position was documented.<br />
The patient received 400 units / kg of<br />
heparin for 24 hours and 200 units / kg<br />
for another day. Antibiotics were<br />
repeated twice during the first 24 hours.<br />
Transthoracic echocardiography showed<br />
a good result with no residual shunt.<br />
The patient was dismissed 48 hours<br />
after the procedure.<br />
Discussion:<br />
This was our first implantation of<br />
<strong>Version</strong> 2.0 of the GORE ® HELEX Septal<br />
Occluder. Several modifications of<br />
the prior version have been made by<br />
W. L. <strong>Gore</strong> & Associates to improve<br />
the handling of the delivery system.<br />
Specifically, the mandrel has been<br />
reinforced by a stainless steel wire to<br />
prevent potential kinking. This issue<br />
intermittently occurred with the previous<br />
version when the distal end of the<br />
mandrel was sharply bent or pushed<br />
forward during the delivery procedure.<br />
The result of a kinked mandrel included<br />
an inability to properly configure the<br />
left-sided disc, thus alleviation of this<br />
potential issue with the new version of<br />
the device is a beneficial improvement.<br />
Despite the mandrel stiffener, it is still<br />
recommended to not push the mandrel<br />
forward during the procedure, which<br />
is under normal circumstances an<br />
unnecessary maneuver.<br />
As a second modification, the gray<br />
control catheter of the improved device<br />
now has a hydrophilic lubricious coating<br />
intended to decrease the frictional<br />
forces within the delivery catheter.<br />
This markedly improves the kinesthetic<br />
feel with which the device may be<br />
deployed. In our case, the decreased<br />
Figure 5: In the first attempt, the device was noted to be unequally deployed<br />
across the septum. The device was completely reloaded into the delivery<br />
catheter and a second attempt was initiated.<br />
Figure 6: Under echocardiography guidance, the left side of the<br />
GORE ® HELEX Device showed a flat configuration and was noted to be<br />
well-apposed to the atrial septum.<br />
Figure 7: Following deployment of the right disc, echocardiography<br />
revealed that the device was in good position, capturing the septal tissue.<br />
No residual shunt was seen.