69-72 - Polskie Stowarzyszenie BiomateriaÅów
69-72 - Polskie Stowarzyszenie BiomateriaÅów
69-72 - Polskie Stowarzyszenie BiomateriaÅów
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
The aim of this study was to develop scaffolds for cartilage<br />
regeneration to be used in reconstructive and plastic<br />
surgery. The porous scaffolds were produced from biocompatible<br />
and resorbable polymer – poly(L-lactide-co-glycolide)<br />
(PLG) acting as a supportive matrix. A thin layer of sodium<br />
hyaluronate (Hyal) was also deposited on the surface as<br />
well in the pore walls of PLG scaffolds in order to provide<br />
biologically active molecules promoting differentiation and<br />
regeneration of the tissue. Physical and chemical properties<br />
of the scaffolds were evaluated and the scaffolds were<br />
implanted in the rabbit auricular cartilage.<br />
Materials and methods<br />
Materials<br />
A copolymer of L-lactide and glycolide (PLG), with the<br />
molar ratio of L-lactide to glycolide 85:15 (molecular masses:<br />
M n =80kDa, M w =152kDa), was synthesised in the Centre<br />
of Polymer and Carbon Materials (Polish Academy of Sciences,<br />
Zabrze, Poland) according to the method described<br />
previously [5].<br />
The PLG scaffolds were produced by solvent casting/<br />
particulate leaching technique. Sieved sodium chloride<br />
particles (POCh, Gliwice, Poland) of defined grain fraction:<br />
250µm-320µm, were mixed with 10% (w/v) copolymer solution<br />
in methylene chloride (POCh, Gliwice, Poland) in such<br />
proportions that a salt volume fraction of 85% was obtained.<br />
The mixture was transferred into polypropylene vials<br />
(diameter 12mm, 5ml volume) and dried overnight in the air,<br />
followed by vacuum treatment at a reduced pressure for 48h.<br />
Next, the vials with the rigid salt/polymer mixture were cut<br />
into slices of the thickness of 2mm and placed into 100ml<br />
of ultra high purity water (UHQ-water, produced by Purelab<br />
UHQ-PS apparatus, Elga, UK). The water was exchanged<br />
several times until the conductivity of the water after washing<br />
was about 2µS/cm, which took 2-3 days. The samples were<br />
then dried in a vacuum oven at 35 o C for <strong>72</strong>h.<br />
The PLG-Hyal scaffolds were produced according to<br />
the following procedure. UHQ-water was used to dissolve<br />
hyaluronic acid (Na salt powder, CPN Spol. s r.o., Czech<br />
Republic) to the concentration of 80µg/ml. Subsequently,<br />
each scaffold was put in 5ml of the solution in the glass vial<br />
and placed in a vacuum oven to apply a vacuum of 0.08MPa<br />
for 10min, in order to impregnate the whole scaffold with<br />
sodium hyaluronian solution. The scaffolds were then dried<br />
in air for 24h followed by drying under vacuum for <strong>72</strong>h, and<br />
stored in a desiccator prior to use.<br />
Materials characterization<br />
The microstructure of the scaffolds was studied with the<br />
use of a scanning electron microscope (Nova NanoSEM<br />
200, FEI, U.S.A.); accelerating voltage 5kV, magnification:<br />
600x, vacuum 60 Pa, without any coating with a conductive<br />
layer.<br />
Fourier transform infrared spectroscopy analysis (FTIR)<br />
was performed in the attenuated total reflection mode (ATR)<br />
with the use of Digilab FTS60 (BioRad).<br />
Implantation<br />
The experiment was performed according to the EU ISO<br />
10993-6 guidelines and the study protocol was approved by<br />
the local bioethics committee in Katowice (No 17/2007, 21<br />
Feb 2007). Before implantation the samples were sterilized<br />
with hydrogen peroxide plasma method (Sterrad 120, ASP,<br />
Johnson & Johnson). Four adult white New Zealand rabbits<br />
were used in the experiment. The animals were operated in<br />
general anaesthesia. Superichondrically 6 mm x 4 mm fragment<br />
of the auricular cartilage was removed and replaced<br />
with the scaffold (6x4x1.5 mm). Each animal received two<br />
implants: PLG or PLG-Hyal scaffolds were implanted into<br />
the right and left ears. The tissues were sutured with polyglycolide<br />
3-0 Safil. After 1 and 4 weeks from the surgery<br />
the animals were painlessly euthanized by an overdose of<br />
Morbital (200 mg/kg body weight).<br />
Histological and histochemical evaluation<br />
Implants and surrounding tissues were excised, frozen<br />
in carbon dioxide ice and subsequently sectioned with<br />
a cryostat microtom into slices 10 µm thick. Obtained slices<br />
were stained by the May-Grünwald Giemsa (MGG) method<br />
to estimate the tissue morphology, number of inflammatory<br />
cells, and degradation process of the scaffolds. The relative<br />
number of inflammatory cells and the activity of hydrolytic<br />
enzyme acid phosphatase (AcP) revealed by Goldberg and<br />
Barka method [6] were used to assess the extent of inflammation<br />
in tissues around the implants.<br />
Observations were made using an optical microscope<br />
(Olympus BH2, objectives 4x and 10x), and pictures were<br />
taken with a digital camera.<br />
Results and discussion<br />
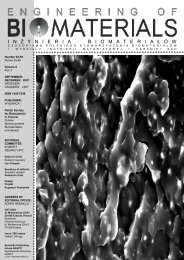
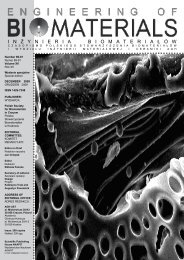
The microstructure of the scaffolds observed under<br />
scanning electron microscope is presented in FIG.1 A, B.<br />
The pore walls of the PLG-Hyal scaffolds (FIG.1B) are much<br />
rough and textured than those of PLG (FIG.1A) because of<br />
the presence of hyaluronate layer on their surface.<br />
Presence of hyaluronate was also proved by infrared spectroscopy<br />
(FTIR-ATR) (FIG. 2 A, B). It can be seen that all peaks<br />
characteristic of poly(L-lactide-co-glycolide) at 1730 cm -1<br />
assigned to C=O and peaks in the range of 1000-1400 cm -1<br />
attributed to C-O and C-O-C stretching vibrations [7] appeared<br />
in both PLG and PLG-Hyal materials. However extra peaks:<br />
at 1530 cm -1 , at 2800 cm -1 and at 3600 cm -1 were observed in<br />
the spectra of PLG-Hyal scaffolds. These peaks attributed<br />
to amide II, C-H, and O-H stretching vibrations, respectively,<br />
are characteristic of hyaluronic acid and hyaluronates [8].<br />
A<br />
B<br />
FIG.1. SEM micrographs of PLG (A) and PLG-Hyal<br />
scaffolds (B)<br />
FIG.2. FTIR-ATR spectra of PLG (a) and PLG-Hyal<br />
scaffolds (b).