Graz University of Technology Austria Institute of Biochemistry ...
Graz University of Technology Austria Institute of Biochemistry ...
Graz University of Technology Austria Institute of Biochemistry ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Graz</strong> <strong>University</strong> <strong>of</strong> <strong>Technology</strong><br />
<strong>Austria</strong><br />
<strong>Institute</strong> <strong>of</strong> <strong>Biochemistry</strong><br />
Annual Report 2010<br />
1
Staff Members <strong>of</strong> the <strong>Institute</strong> <strong>of</strong> <strong>Biochemistry</strong>, TU <strong>Graz</strong><br />
http://www.biochemistry.tugraz.at/<br />
Pr<strong>of</strong>essors<br />
Peter Macheroux (Full Pr<strong>of</strong>essor & Head <strong>of</strong> the <strong>Institute</strong>)<br />
peter.macheroux@tugraz.at; Tel.: +43-(0)316-873-6450<br />
Günther Daum (Associate Pr<strong>of</strong>essor)<br />
guenther.daum@tugraz.at; Tel.: +43-(0)316-873-6462<br />
Albin Hermetter (Associate Pr<strong>of</strong>essor)<br />
albin.hermetter@tugraz.at; Tel.: +43-(0)316-873-6457<br />
Michael Murkovic (Associate Pr<strong>of</strong>essor)<br />
michael.murkovic@tugraz.at; Tel.: +43-(0)316-873-6495<br />
Karin Athenstaedt (Independent Group Leader)<br />
karin.athenstaedt@tugraz.at; Tel.: +43-(0)316-873-6460<br />
Assistants<br />
Ines Waldner-Scott<br />
ines.waldner-scott@tugraz.at; Tel.: +43-(0)316-873-6454<br />
Tanja Knaus<br />
tanja.knaus@tugraz.at; Tel.: +43-(0)316-873-6463<br />
Alexandra Binter<br />
alexandra.binter@tugraz.at; Tel.: +43-(0)316-873-6453<br />
Silvia Wallner<br />
silvia.wallner@tugraz.at; Tel.: +43-(0)316-873-6955<br />
Office<br />
Annemarie Portschy<br />
portschy@tugraz.at; Tel.: +43-(0)316-873-6451; Fax: +43-(0)316-873-6952<br />
Technical Staff<br />
Claudia Hrastnik; claudia.hrastnik@tugraz.at; Tel.: +43-(0)316-873-6460<br />
Steve Stipsits; steve.stipsits@tugraz.at; Tel.: +43-(0)316-873-6464<br />
Rosemarie Trenker-El-Toukhy; r.trenker-el-toukhy@tugraz.at; Tel.: +43-(0)316-873-6464<br />
Elfriede Zenzmaier; elfriede.zenzmaier@tugraz.at; Tel.: +43-(0)316-873-6467<br />
Alma Ljubijankic; alma.ljubijankic@tugraz.at; Tel.: +43-(0)316-873-6460 or 6498<br />
Eva Maria Pointner; eva.pointner@tugraz.at; Tel.: +43-(0)316-873-6453<br />
Leo H<strong>of</strong>er (Workshop); leo.h<strong>of</strong>er@tugraz.at; Tel.: +43-(0)316-873-8431 or 8433<br />
2
Brief History <strong>of</strong> the <strong>Institute</strong> <strong>of</strong> <strong>Biochemistry</strong><br />
The <strong>Institute</strong> <strong>of</strong> <strong>Biochemistry</strong> and Food Chemistry was born out <strong>of</strong> the division <strong>of</strong> the <strong>Institute</strong><br />
<strong>of</strong> Biochemical <strong>Technology</strong>, Food Chemistry and Microchemistry <strong>of</strong> the former School <strong>of</strong><br />
<strong>Technology</strong> <strong>Graz</strong>. Together with all the other chemistry institutes, it was located in the old<br />
Chemistry Building on Baron Mandell's ground, corner Technikerstrasse-Mandellstrasse.<br />
1929 The <strong>Institute</strong> <strong>of</strong> Technical <strong>Biochemistry</strong> and Microbiology moved to the building <strong>of</strong><br />
the Fürstlich-Dietrichstein-Stiftung, Schlögelgasse 9, in which all the biosciences were<br />
then concentrated.<br />
1945 G. GORBACH - initially in the rank <strong>of</strong> a docent and soon thereafter as a.o. Pr<strong>of</strong>essor -<br />
took over to lead the institute. The institute was renamed <strong>Institute</strong> <strong>of</strong> Biochemical<br />
<strong>Technology</strong> and Food Chemistry.<br />
1948 G. GORBACH was nominated full pr<strong>of</strong>essor and head <strong>of</strong> the institute. In succession <strong>of</strong><br />
the famous <strong>Graz</strong> School <strong>of</strong> Microchemistry founded by Pr<strong>of</strong>. F. PREGL and Pr<strong>of</strong>. F.<br />
EMICH, Pr<strong>of</strong>. GORBACH was one <strong>of</strong> the most prominent and active leaders in the<br />
fields <strong>of</strong> microchemistry, microbiology and nutritional sciences. After World War II,<br />
questions <strong>of</strong> water quality and waste water disposal became urgent; hence, the group<br />
<strong>of</strong> Pr<strong>of</strong>. K. STUNDL, which at that time was part <strong>of</strong> the institute, was gaining<br />
importance. In addition, a division to fight dry-rot supervised by Dr. KUNZE and after<br />
his demise by H. SALOMON, was also affiliated with the institute.<br />
1955 In honour <strong>of</strong> the founder <strong>of</strong> microchemistry and former pr<strong>of</strong>essor at the <strong>Graz</strong><br />
<strong>University</strong> <strong>of</strong> <strong>Technology</strong>, the extended laboratory was called EMICH-Laboratories.<br />
At the same time, the institute was renamed <strong>Institute</strong> <strong>of</strong> Biochemical <strong>Technology</strong>, Food<br />
Chemistry and Microchemistry.<br />
Lectures and laboratory courses were held in biochemistry, biochemical technology, food<br />
chemistry and food technology, technical microscopy and microchemistry. In addition, the<br />
institute covered technical microbiology together with biological and bacteriological analysis<br />
- with the exception <strong>of</strong> pathogenic microorganisms - and a lecture in organic raw materials<br />
sciences.<br />
1970 After the decease <strong>of</strong> Pr<strong>of</strong>. GORBACH, Pr<strong>of</strong>. GRUBITSCH was appointed head <strong>of</strong> the<br />
institute. Towards the end <strong>of</strong> the sixties, the division for water and waste water<br />
disposal headed by Pr<strong>of</strong>. STUNDL was drawn out <strong>of</strong> the institute and established as an<br />
independent institute. Pr<strong>of</strong>. SPITZY was nominated pr<strong>of</strong>essor <strong>of</strong> general chemistry,<br />
micro- and radiochemistry. This division was also drawn out <strong>of</strong> the mother institute<br />
and at the end <strong>of</strong> the sixties moved to a new building.<br />
1973 Division <strong>of</strong> the <strong>Institute</strong> for Biochemical <strong>Technology</strong>, Food <strong>Technology</strong> and<br />
Microchemistry took place. At first, biochemical technology together with food<br />
technology formed a new institute now called <strong>Institute</strong> <strong>of</strong> Biotechnology and Food<br />
Chemistry headed by Pr<strong>of</strong>. LAFFERTY.<br />
1973 Dr. F. PALTAUF, docent at the Karl-Franzens-<strong>University</strong> <strong>Graz</strong>, was appointed<br />
pr<strong>of</strong>essor and head <strong>of</strong> the newly established <strong>Institute</strong> <strong>of</strong> <strong>Biochemistry</strong>. The interest <strong>of</strong><br />
Pr<strong>of</strong>. PALTAUF in studying biological membranes and lipids laid the foundation for<br />
the future direction <strong>of</strong> research. G. DAUM, S. D. KOHLWEIN, and A. HERMETTER<br />
3
joined the institute. All three young scientists were given the chance to work as post<br />
docs in renown laboratories in Switzerland and the USA: G. DAUM with the groups<br />
<strong>of</strong> G. Schatz (Basel, Switzerland) and R. Schekman (Berkeley, USA), A.<br />
HERMETTER with J. R. Lakowicz (Baltimore, USA) and S. D. KOHLWEIN with S.<br />
A. Henry (New York, USA). Consequently, independent research groups specialized<br />
in cell biology (G. D.), biophysics (A. H.) and molecular biology (S. D. K.) evolved at<br />
the institute in <strong>Graz</strong>, with the group <strong>of</strong> Pr<strong>of</strong>. F. PALTAUF still focusing on the<br />
chemistry and biochemistry <strong>of</strong> lipids.<br />
Teaching was always a major task <strong>of</strong> the institute. Lectures, seminars and laboratory courses<br />
in basic biochemistry were complemented by special lectures, seminars, and courses held by<br />
the assistants who became docents in 1985 (G. D.), 1987 (A. H.), and 1992 (S. D. K.).<br />
Lectures in food chemistry and technology were held by C. WEBER and H. SALOMON.<br />
Hence the institute was renamed <strong>Institute</strong> <strong>of</strong> <strong>Biochemistry</strong> and Food Chemistry.<br />
1990 The institute moved to a new building at Petersgasse 12. The move was accompanied<br />
by the expansion <strong>of</strong> individual research groups and the acquisition <strong>of</strong> new equipment<br />
essential for the participation in novel collaborative efforts at the national and<br />
international level. Thus, the <strong>Institute</strong> <strong>of</strong> <strong>Biochemistry</strong>, together with partner institutes<br />
from the Karl-Franzens <strong>University</strong> was the driving force to establish <strong>Graz</strong> as a centre<br />
<strong>of</strong> competence in lipid research.<br />
1993 W. PFANNHAUSER was appointed as pr<strong>of</strong>essor <strong>of</strong> food chemistry. Through his own<br />
enthusiasm and engagement and that <strong>of</strong> his collaborators, this new section <strong>of</strong> the<br />
institute rapidly developed and <strong>of</strong>fered students additional opportunities to receive a<br />
timely education.<br />
2000 The two sections, biochemistry and food chemistry, being independent <strong>of</strong> each other<br />
with respect to personnel, teaching, and research, were separated into the <strong>Institute</strong> <strong>of</strong><br />
<strong>Biochemistry</strong> (Head Pr<strong>of</strong>. PALTAUF) and the new <strong>Institute</strong> <strong>of</strong> Food Chemistry and<br />
<strong>Technology</strong> (Head Pr<strong>of</strong>. PFANNHAUSER).<br />
2001 After F. PALTAUF’s retirement, in September 2001, G. DAUM was elected head <strong>of</strong><br />
the institute. S. D. KOHLWEIN was appointed full pr<strong>of</strong>essor <strong>of</strong> biochemistry at the<br />
Karl-Franzens <strong>University</strong> <strong>Graz</strong>.<br />
2003 P. MACHEROUX was appointed full pr<strong>of</strong>essor <strong>of</strong> biochemistry in September 2003<br />
and head <strong>of</strong> the <strong>Institute</strong> <strong>of</strong> <strong>Biochemistry</strong> in January 2004. His research interests<br />
revolve around topics in protein biochemistry and enzymology and shall strengthen the<br />
already existing activities in this area.<br />
2007 K. ATHENSTAEDT, a long-time associate <strong>of</strong> Pr<strong>of</strong>. DAUM, received the venia<br />
legendi for biochemistry. Dr. Athenstaedt was the first woman to complete the<br />
traditional habilitation at the <strong>Institute</strong> <strong>of</strong> <strong>Biochemistry</strong>.<br />
2009 After the retirement <strong>of</strong> Pr<strong>of</strong>. PFANNHAUSER in 2008, the <strong>Institute</strong> <strong>of</strong> Food<br />
Chemistry and <strong>Technology</strong> was disbanded and the research group <strong>of</strong> Pr<strong>of</strong>. M.<br />
MURKOVIC joined the <strong>Institute</strong> <strong>of</strong> <strong>Biochemistry</strong> increasing the number <strong>of</strong><br />
independent research groups to five.<br />
4
Highlights <strong>of</strong> 2010<br />
In 2010, Peter Macheroux took a break from his teaching duties and spent most <strong>of</strong> the year<br />
from mid-February until the end <strong>of</strong> July on sabbatical leave in the Dept. <strong>of</strong> Chemistry &<br />
Chemical Biology at Cornell <strong>University</strong> (hosted by Pr<strong>of</strong>. Steve Ealick). This was a truly<br />
unique experience not just because <strong>of</strong> the opportunity to acquire insight into new scientific<br />
methods but also in terms <strong>of</strong> getting to know new colleagues and customs, such as<br />
commencement day where students obtain their academic degree during a traditional<br />
celebration. In spring 2010 the <strong>Austria</strong>n Science Fund (FWF) approved a new research project<br />
to investigate the interaction <strong>of</strong> quinone reductase with the proteasome that controls the<br />
localization <strong>of</strong> some transcription factors (Project P22361: “Mechanism <strong>of</strong> redox controlled<br />
protein degradation”). Finally, Dr. Andreas Winkler, a former PhD student <strong>of</strong> the PhD<br />
program “Molecular Enzymology” and currently a postdoctoral fellow at the Max-Planck<br />
<strong>Institute</strong> <strong>of</strong> Molecular Medicine in Heidelberg received the dissertation award 2010 <strong>of</strong> the<br />
<strong>Austria</strong>n Society for Molecular Biosciences and Biotechnology (ÖGMBT) for the thesis he<br />
has completed in our institute the year before.<br />
Impressions from the sabbatical <strong>of</strong> P. Macheroux<br />
Commencement day at Cornell in May 2010<br />
Karlheinz Grillitsch was the 100 th student to receive<br />
his PhD at the <strong>Institute</strong> <strong>of</strong> <strong>Biochemistry</strong>. Opponents<br />
were K. Lohner (left) and G. Daum (right <strong>of</strong> the<br />
candidate); chairman A. Hermetter (right).<br />
In the group <strong>of</strong> Günther Daum, three new projects were started in 2010. Whereas the FWF<br />
project P23029 is devoted to the fundamental investigation <strong>of</strong> yeast lipases, the Translational<br />
Research Project TRP009 addresses applied aspects <strong>of</strong> Pichia lipidomics. Finally, the lab <strong>of</strong><br />
Günther Daum is involved in investigations <strong>of</strong> protein expression in the framework <strong>of</strong> the<br />
<strong>Austria</strong>n Centre <strong>of</strong> Industrial Biotechnology (ACIB). A highlight in graduate student<br />
education was the completion <strong>of</strong> the Doctoral Thesis <strong>of</strong> Karlheinz Grillitsch because this was<br />
the 100 th PhD degree received at the <strong>Institute</strong> <strong>of</strong> <strong>Biochemistry</strong>, TU <strong>Graz</strong>. In 2010, Günther<br />
Daum started his three year term as President <strong>of</strong> the International Conference on the<br />
Bioscience <strong>of</strong> Lipids (ICBL). In May 2010, he was Organizer and Chairman <strong>of</strong> the FEBS<br />
Workshop Microbial Lipids: From Genomics to Lipidomics, in Vienna, <strong>Austria</strong>. This was the<br />
first conference <strong>of</strong> this type, but due to its success with more than 100 participants the next<br />
meeting has already be planned for 2012 in Bern, Switzerland.<br />
5
In 2010 Karin Athenstaedt continued her work in our institute as an independent<br />
researcher. With her project she set the cornerstone to establish her own research group.<br />
Karin Athenstaedt’s work is devoted to lipid metabolism in yeast.<br />
Basic research in Albin Hermetter’s group was performed in three projects focusing on lipid<br />
(patho)physiology. His contribution to the GOLD project (speaker R. Zechner, ZMB,<br />
<strong>University</strong> <strong>of</strong> <strong>Graz</strong>, funded by the GEN-AU program <strong>of</strong> the BM.W_F) is devoted to the<br />
functional proteomic analysis <strong>of</strong> (phospho)lipases relevant to lipid-associated disorders. In the<br />
SFB LIPOTOX (FWF project, speaker R. Zechner) and the EuroMEMBRANE consortium<br />
OXPHOS (speaker P. Kinnunen, Aalto <strong>University</strong>, Helsinki), A. Hermetter studies the toxicity<br />
<strong>of</strong> oxidized phospholipids in the cells <strong>of</strong> the arterial wall (funded by FWF). Research in this<br />
field is also supported by the PhD program “Molecular Enzymology” (FWF). Ute Stemmer<br />
who is a PhD student in the SFB LIPOTOX was awarded the prize for the best oral<br />
presentation by young researchers entitled “Oxidized phospholipids: uptake and targeting in<br />
RAW 264.7 macrophages” at the International Conference on the Bioscience <strong>of</strong> Lipids in<br />
Bilbao, Spain, September 7 – 11, 2010.<br />
K. Athenstaedt<br />
M. Murkovic<br />
Ute Stemmer (in the center) received the BBA Award presented by W.<br />
Dowhan (left; BBA Executive Editor) for the best oral presentation by young<br />
researchers at the International Conference on the Bioscience <strong>of</strong> Lipids in<br />
Bilbao, Spain, September 7 – 11, 2010.<br />
In 2010, Alan Zeb finished his PhD Thesis in the group “Chemistry <strong>of</strong> Functional Foods”<br />
directed by Michael Murkovic in which the interaction <strong>of</strong> carotenoids with triglycerides from<br />
edible oils was characterized in detail. The pro- and antioxidant action <strong>of</strong> carotenoids was<br />
evaluated by a detailed analysis <strong>of</strong> the oxidation products by LC-MS. Several oxidation<br />
products <strong>of</strong> triglycerides were newly identified. Four manuscripts were published on this<br />
topic. In 2010 the biannual conference <strong>of</strong> the <strong>Austria</strong>n Food Chemists was held at Schloss<br />
Seggau near Leibnitz. At this conference around 100 participants from <strong>Austria</strong> and the<br />
neighboring countries discussed the issue <strong>of</strong> food quality on the molecular level.<br />
6
<strong>Biochemistry</strong> group<br />
Group leader: Peter Macheroux<br />
Secretary: Annemarie Portschy<br />
Postdoctoral Fellow: Ines Waldner-Scott<br />
PhD students: Thomas Bergner, Alexandra Binter, Venugopal Gudipati, Tanja Knaus, Wolf-<br />
Dieter Lienhart, Silvia Wallner<br />
Technicians: Eva Maria Pointner, Steve Stipsits, Rosemarie Trenker-El-Toukhy<br />
Alumni 2010: Katrin Fantur (PhD)<br />
General description<br />
The fundamental questions in the study <strong>of</strong> enzymes, the bio-catalysts <strong>of</strong> all living organisms,<br />
revolve around their ability to select a substrate (substrate specificity) and subject this<br />
substrate to a predetermined chemical reaction (reaction and regio-specificity). In general,<br />
only a few amino acid residues in the "active site" <strong>of</strong> an enzyme are involved in this process<br />
and hence provide the key to the processes taking place during enzyme catalysis. Therefore,<br />
the focus <strong>of</strong> our research is to achieve a deeper understanding <strong>of</strong> the functional role <strong>of</strong> amino<br />
acids in the active site <strong>of</strong> enzymes with regard to substrate-recognition and stereo- and<br />
regiospecificity <strong>of</strong> the chemical transformation. In addition, we are also interested in<br />
substrate-triggered conformational changes and how enzymes utilize c<strong>of</strong>actors (flavin,<br />
nicotinamide) to achieve catalysis. Towards these aims we employ a multidisciplinary<br />
approach encompassing kinetic, thermodynamic, spectroscopic and structural techniques. In<br />
addition, we use site-directed mutagenesis to generate mutant enzymes to probe their<br />
functional role in the mentioned processes. Furthermore, we collaborate with our partners in<br />
academia and industry to develop inhibitors for enzymes, which can yield important new<br />
insights into enzyme mechanisms and can be useful as potential lead compounds in the design<br />
<strong>of</strong> new drugs.<br />
The methods established in our laboratory comprise kinetic (stopped-flow and rapid quench<br />
analysis <strong>of</strong> enzymatic reactions), thermodynamic (isothermal titration microcalorimetry) and<br />
spectroscopic (fluorescence, circular dichroism and UV/VIS absorbance) methods. We also<br />
frequently use MALDI-TOF and ESI mass spectrometry, protein purification techniques<br />
(chromatography and electrophoresis) and modern molecular biology methods to clone and<br />
express genes <strong>of</strong> interest. A brief description <strong>of</strong> our current research projects is given below.<br />
Berberine bridge enzyme & other flavin-dependent plant oxidases<br />
Berberine bridge enzyme (BBE) is a central enzyme in the biosynthesis <strong>of</strong> berberine, a<br />
pharmaceutically important alkaloid. The enzyme possesses a covalently attached FAD<br />
moiety, which is essential for catalysis. The reaction involves the oxidation <strong>of</strong> the N-methyl<br />
group <strong>of</strong> the substrate (S)-reticuline by the enzyme-bound flavin and concomitant formation<br />
<strong>of</strong> a carbon-carbon bond (the “bridge”). The ultimate acceptor <strong>of</strong> the substrate-derived<br />
electrons is dioxygen, which reoxidizes the flavin to its resting state:<br />
7
The BBE-catalysed oxidative carbon-carbon bond formation is a new example <strong>of</strong> the<br />
versatility <strong>of</strong> the flavin c<strong>of</strong>actor in biochemical reactions. Our goal is to understand the<br />
oxidative cyclization reaction by a biochemical and structural approach.<br />
We have developed a new expression system for BBE (using cDNA from Eschscholzia<br />
california, gold poppy) in Pichia pastoris, which produces large amounts <strong>of</strong> the protein (ca.<br />
500 mg from a 10-L culture). The availability <strong>of</strong> suitable quantities <strong>of</strong> BBE enabled us to<br />
crystallize the protein and to solve the structure in collaboration with Pr<strong>of</strong>. Karl Gruber at the<br />
Karl-Franzens <strong>University</strong> <strong>Graz</strong> (see below).<br />
Based on the three-dimensional structure <strong>of</strong> BBE, we have performed a site-directed<br />
mutagenesis program to investigate the role <strong>of</strong> amino acids present in the active site <strong>of</strong> the<br />
enzyme. In conjunction with other experiments, this has led to the formulation <strong>of</strong> a new<br />
reaction mechanism for the enzyme (thesis project <strong>of</strong> Andreas Winkler). Currently, Silvia<br />
Wallner investigates alternative covalent modifications in the 8α-position in BBE variants<br />
that contain either aspartate or tyrosine instead <strong>of</strong> a histidine. In collaboration with Pr<strong>of</strong>. Toni<br />
Kutchan at the Donald Danforth Plant Science Center in St. Louis, we have identified other<br />
8
plant genes that apparently encode flavin-dependent oxidases. These genes are currently<br />
expressed in our laboratory in order to characterize the role <strong>of</strong> the enzymes in alkaloid<br />
biosynthesis (thesis project <strong>of</strong> Silvia Wallner).<br />
Dipeptidylpeptidase III<br />
Dipeptidyl-peptidases III (DPPIII; EC 3.4.14.4) are Zn-dependent enzymes with molecular<br />
masses <strong>of</strong> ca. 80-85 kDa that specifically cleave the first two amino acids from the N-<br />
terminus <strong>of</strong> different length peptides. All known DPPIII sequences contain the unique motif<br />
HEXXGH, which enabled the recognition <strong>of</strong> the dipeptidyl-peptidase III family as a distinct<br />
evolutionary metallopeptidase family (M49). In mammals, DPPIII is associated with<br />
important physiological functions such as pain regulation, and hence the enzyme is a potential<br />
drug target. Previously, Sigrid Deller and Nina Jajcanin-Jozic have successfully expressed,<br />
purified and characterized the recombinant yeast enzyme, and Pravas Baral in Karl Gruber’s<br />
laboratory at the Karl-Franzens-<strong>University</strong> has elucidated the crystal structure <strong>of</strong> the yeast<br />
protein. This work revealed that yeast DPPIII features a novel protein topology.<br />
Structure <strong>of</strong> human DPPIII in its open form (right) and peptide liganded (closed) form<br />
In collaboration with a structural genomics group in Toronto led by Dr. Sirano Dhe-Paganon,<br />
Gustavo Arruda has solved the structure <strong>of</strong> the human enzyme both in its open (right, above)<br />
and closed conformation (left, above). The latter structure was obtained by co-crystallization<br />
<strong>of</strong> a tightly binding peptide to an inactive variant <strong>of</strong> human DPPIII. These two new structures<br />
constitute a major breakthrough in our effort to understand the physiological role <strong>of</strong> the<br />
enzyme and pave the way for the development <strong>of</strong> potentially useful inhibitors <strong>of</strong> the enzyme<br />
(Gustavo Arruda’s thesis project in Pr<strong>of</strong>. Gruber’s laboratory supported by Alexandra Binter).<br />
9
Luciferase and LuxF<br />
The emission <strong>of</strong> light by biological species (bioluminescence) is a fascinating process found<br />
in diverse organisms such as bacteria, funghi, insects, fish, limpets and nematodes. In all cases<br />
the bioluminescent process is based on a chemiluminescent reaction in which the chemical<br />
energy is (partially) transformed into light energy ("cold light"). All bioluminescent processes<br />
require a luciferase, i.e. an enzyme catalyzing the chemiluminescent reaction, and a luciferin,<br />
which can be considered a coenzyme. During the bioluminescent reaction the luciferin is<br />
generated in an excited state and serves as the emitter <strong>of</strong> light energy. In our laboratory, we<br />
are interested in the bioluminescence <strong>of</strong> marine photobacteria. In these bacteria, luciferase is<br />
composed <strong>of</strong> an alpha/beta-heterodimeric protein, which binds reduced flavinmononucleotide<br />
(FMN) as the luciferin. The reduced FMN reacts with molecular dioxygen to a hydroperoxide<br />
intermediate with subsequent oxidation <strong>of</strong> a long-chain fatty aldehyde (e.g. tetradecanal) to<br />
the corresponding fatty acid (e.g. myristic acid). During this oxidation process, an excited<br />
flavin intermediate is generated which emits light. Some marine photobacteria possess an<br />
additional protein called LuxF which was found in complex with a myristylated flavin<br />
derivative where the C-3 atom <strong>of</strong> myristic acid is covalently attached to the 6-position <strong>of</strong> the<br />
flavin ring system. It was postulated that this flavin adduct is generated in the luciferase<br />
catalyzed bioluminescent reaction. Furthermore, it was speculated that LuxF sequesters the<br />
myristylated flavin adduct in order to prevent inhibition <strong>of</strong> the bioluminescent reaction.<br />
However, both hypotheses have not been tested on a biochemical or physiological level yet.<br />
Hence, in this study we will design and perform experiments to examine the putative<br />
generation <strong>of</strong> myristylated FMN through the luciferase reaction (thesis project <strong>of</strong> Thomas<br />
Bergner supported by Steve Stipsits)<br />
Structure <strong>of</strong> a LuxF dimer in the absence (red) and presence (blue) <strong>of</strong> the myristylated flavin<br />
derivative (pdb code 1NFP)<br />
Nikkomycin biosynthesis<br />
Nikkomycins are produced by several species <strong>of</strong> Streptomyces and exhibit fungicidal,<br />
insecticidal and acaricidal properties due to their strong inhibition <strong>of</strong> chitin synthase.<br />
Nikkomycins are promising compounds in the cure <strong>of</strong> the immunosuppressed, such as AIDS<br />
patients, organ transplant recipients and cancer patients undergoing chemotherapy.<br />
Nikkomycin Z (R 1 = uracil & R 2 = OH, see below) is currently in clinical trial for its antifungal<br />
activity. Structurally, nikkomycins can be classified as peptidyl nucleosides containing two<br />
10
unusual amino acids, i.e. hydroxypyridylhomothreonine (HPHT) and aminohexuronic acid<br />
with an N-glycosidically linked base:<br />
Although the chemical structures <strong>of</strong> nikkomycins have been known since the 1970s, only a<br />
few biosynthetic steps have been investigated in detail. The steps leading to the synthesis <strong>of</strong><br />
aminohexuronic acid are unclear. Originally, it was hypothesized that the aminohexuronic<br />
acid moiety is generated by addition <strong>of</strong> an enolpyruvyl moiety from phosphoenolpyruvate<br />
(PEP) to either the uridine or the 4-formyl-4-imidazolin-2-one analog at the 5’-position <strong>of</strong><br />
ribose. This step is then followed by rather speculative modifications to yield the<br />
aminohexuronic acid precursor. In contrast to this hypothesis, we could recently demonstrate<br />
that UMP rather than uridine serves as the acceptor for the enolpyruvyl moiety, a reaction<br />
catalyzed by an enzyme encoded by a gene <strong>of</strong> the nikkomycin operon termed nikO.<br />
Furthermore, we could demonstrate that it is attached to the 3’- rather than the 5’-position <strong>of</strong><br />
UMP. These results are very intriguing since none <strong>of</strong> the nikkomycins synthesized possess an<br />
enolpyruvyl group in this position <strong>of</strong> the sugar moiety. Hence, it must be concluded that the<br />
resulting 3’-enolpyruvyl-UMP is subject to rearrangement reactions where the enolpyruvyl is<br />
detached from its 3’-position and transferred to the 5’-position <strong>of</strong> the ensuing aminohexuronic<br />
acid moiety. Co-crystallization <strong>of</strong> NikO with fosfomycin yielded rod – like crystals diffracting<br />
up to 2.5 Å. A synchrotron dataset was measured at the Swiss Light Source and the structure<br />
was solved by molecular replacement using UDP-N-acetylglucosamine enolpyruvyl<br />
transferase (PDB code: 2rl1) as a model. Two molecules were found in the asymmetric unit<br />
exhibiting an inverse α,β-barrel fold with helices forming the tightly packed core and sheets<br />
shielding the hydrophobic core from the solvent:<br />
Eech chain is comprised <strong>of</strong> two inverse α,β-barrel subunits, which are connected by a hinge<br />
region. The final structure was refined to final R/R free values <strong>of</strong> 17% and 19%, respectively.<br />
Crystallization trials <strong>of</strong> NikO in the presence <strong>of</strong> its product, 3’-EP-UMP, are currently under<br />
way.<br />
11
The genes that are co-transcribed with nikO have been reported and are designated as nikI,<br />
nikJ, nikK, nikL, nikM and nikN. In order to investigate the role <strong>of</strong> the encoded proteins in the<br />
biosynthesis <strong>of</strong> the aminohexuronic acid moiety, these genes are cloned, expressed and the<br />
proteins purified for biochemical characterization and crystallization (thesis project <strong>of</strong><br />
Alexandra Binter and Gustav Oberdorfer in Pr<strong>of</strong>. Gruber’s laboratory).<br />
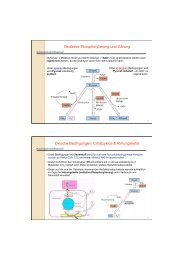
Lot6p – a redox regulated switch <strong>of</strong> the proteasome<br />
During our previous studies <strong>of</strong> bacterial quinone reductases, we have also investigated the<br />
biochemical properties <strong>of</strong> the yeast homolog Lot6p. Despite the availability <strong>of</strong> a threedimensional<br />
structure for Lot6p (1T0I), the physiological role <strong>of</strong> the enzyme was unclear.<br />
Our recent studies have now demonstrated that the enzyme rapidly reduces quinones at the<br />
expense <strong>of</strong> a reduced nicotinamide c<strong>of</strong>actor, either NADH or NADPH. In order to further<br />
characterize the cellular role <strong>of</strong> Lot6p, we have carried out pull-down assays and identified<br />
the 20S core particle <strong>of</strong> the yeast proteasome as interaction partner. Further studies revealed<br />
that this complex recruits Yap4p, a member <strong>of</strong> the b-Zip transcription factor family, but only<br />
when the flavin-c<strong>of</strong>actor <strong>of</strong> Lot6p is in its reduced state. Oxidation <strong>of</strong> the flavin leads to<br />
dissociation <strong>of</strong> the transcription factor and relocalization to the nucleus (see scheme below).<br />
reduced<br />
Yap4p<br />
oxidized<br />
Yap4p<br />
Yap4p<br />
Lot6p<br />
α<br />
β<br />
β<br />
α<br />
Oxidation by<br />
e.g. quinones<br />
Very slow with oxygen O 2<br />
oxygen<br />
Lot6p<br />
α<br />
β<br />
β<br />
α<br />
nucleus<br />
A similar system is known from mammalian cells, where a homologous quinone reductase<br />
(NQO1) binds to the 20S proteasome and recruits important tumor suppressor proteins such as<br />
p53 and p73α. Hence, the discovery <strong>of</strong> a homologous protein interaction in yeast provides an<br />
interesting model system to investigate the molecular basis for protein complex formation and<br />
regulation <strong>of</strong> proteasomal degradation <strong>of</strong> transcription factors (postdoctoral project <strong>of</strong> Ines<br />
Waldner-Scott; thesis project <strong>of</strong> Wolf-Dieter Lienhart and Venugopal Gudipati).<br />
Zn-dependent Alkylsulfatases<br />
Hydrolysis <strong>of</strong> alkylsulfates is an important pathway for soil and other bacteria to mobilize<br />
sulfur. Three classes <strong>of</strong> sulfatases - divided according to their reaction mechanism - have been<br />
discovered, and the recently elucidated structure <strong>of</strong> SdsA1 from Pseudomonas aeruginosa is<br />
another example <strong>of</strong> the widely occurring family <strong>of</strong> metallo-ß-lactamases. This group <strong>of</strong><br />
alkylsulfatases is characterized by two Zn 2+ atoms in the active site which activate a water<br />
molecule for nucleophilic attack on the sulfate group. SdsA1 mainly cleaves long-chain<br />
primary alkylsulfates (preferred substrate is dodecylsulfate) by stereoinversion. In other<br />
12
words, the hydroxyl group attacks the carbon atom in the course <strong>of</strong> the reaction. Recently, a<br />
novel enzyme could be identified in Pseudomonas DSM 6611 (termed PISA1 = Pseudomonas<br />
inverting alkylsulfatase 1) which mainly cleaves secondary alkylsulfates, for example 2-<br />
octylsulfate exhibiting stereopreference for the (R)-stereoisomer. In contrast to the majority <strong>of</strong><br />
hydrolases, which do not alter the stereochemistry <strong>of</strong> the substrate during catalysis, PISA1 is<br />
an attractive enzyme for the deracemisation <strong>of</strong> sec-alcohols.<br />
Analysis <strong>of</strong> the crystal structures <strong>of</strong> PISA1 and SdsA1 showed that the overall structure <strong>of</strong><br />
both proteins is virtually identical and both enzymes largely share the same active-site<br />
architecture, such as a sulfate binding site (composed <strong>of</strong> two Arg), a nucleophile site<br />
composed <strong>of</strong> a binuclear Zn 2+ -cluster typical for metallo-ß-lactamases and an Asn/Thrhydrogen<br />
binding network for substrate positioning. However, the active site <strong>of</strong> PISA1<br />
features several conspicuous amino acid exchanges (see figure below: in blue active side<br />
residues in SdsA1 and green those in PISA1).<br />
Phe/Gly<br />
Tyr/His<br />
Met/Ser<br />
Met/Ser<br />
Leu/Pro<br />
Ala/Ile<br />
Tyr/Ser<br />
Tyr/Ser<br />
Ala/Ile<br />
These amino acids are now subject <strong>of</strong> an extensive mutagenesis program to define their role in<br />
governing substrate preference <strong>of</strong> the reaction. This project is a close collaboration with Pr<strong>of</strong>s.<br />
Faber (biocatalysis) and Wagner (structure determination) from the <strong>University</strong> <strong>of</strong> <strong>Graz</strong> (thesis<br />
project <strong>of</strong> Tanja Knaus in our laboratory and Markus Schober in Pr<strong>of</strong>. Faber’s laboratory).<br />
Doctoral thesis completed<br />
Katrin Fantur: Friend or Foe: Iminosugars as inhibitors and pharmacological chaperones <strong>of</strong><br />
the human lysosomal acid β-galactosidase<br />
G M1 -gangliosidosis (GM1) and Morquio B disease (MBD) are rare, hereditary lysosomal<br />
storage disorders caused by mutations in the gene GLB1. Its main gene product, human<br />
lysosomal acid β-galactosidase (β-Gal) degrades N-linked oligosaccharides present in<br />
glycoproteins, GM1-gangliosides in the brain, and keratan sulfate in connective tissues. While<br />
GM1 is a phenotypically heterogenous neurodegenerative disorder, MBD is a systemic bone<br />
disease without effects on the central nervous system. Some mutations in the GLB1 gene<br />
produce stable β-Gal precursors, normally transported and processed to mature,<br />
intralysosomal β-Gal, while others affect precursor stability and intracellular transport<br />
resulting in premature protein degradation. Several misfolded enzymes were shown to be<br />
13
sensitive to stabilization by iminosugars, which bind at the active site to provide the proper<br />
conformation. Thus the stabilized protein may escape from degradation processes, and reach<br />
the lysosomes in an active state, as proposed for enzyme enhancement therapy (EET).<br />
In this work the influence <strong>of</strong> novel derivatives <strong>of</strong> 1-deoxygalactonojirimycin (DGJ)<br />
on the β-Gal activity <strong>of</strong> cultured GM1 and MBD skin fibroblasts was examined. Furthermore,<br />
the effect <strong>of</strong> selected compounds on natural substrate degradation in GM1 and MBD cells was<br />
determined. Several novel iminosugars acting as pharmacological chaperones <strong>of</strong> β-Gal in<br />
specific GM1 fibroblasts were discovered and described in this work. One specific compound,<br />
DLHex-DGJ, proved to be a potent competitive inhibitor <strong>of</strong> β-Gal in vitro, and this work<br />
describes its effects on activity, protein expression, maturation and intracellular transport in<br />
vivo in 13 fibroblast lines with GLB1 mutations. DLHex-DGJ significantly increased the<br />
catalytic activity in six GM1 cell lines, and normalization <strong>of</strong> transport and lysosomal<br />
processing <strong>of</strong> β-Gal precursors was demonstrated for selected cell lines. Furthermore,<br />
DLHex-DGJ and another, similar compound successfully reduced the level <strong>of</strong> internalized<br />
radiolabeled G M1 -gangliosides in a specific GM1 cell line, suggesting that reduction <strong>of</strong> stored<br />
material is possible under certain conditions.<br />
Specific antibodies, directed against human β-Gal, were developed with the aid <strong>of</strong><br />
previously published protocols, and novel approaches to obtain large amounts <strong>of</strong> the purified<br />
human enzyme were tested. Two novel polyclonal anti-β-Gal peptide antibodies were<br />
produced and expression <strong>of</strong> human β-Gal in E. coli cells may provide the basis for further<br />
development <strong>of</strong> antibodies directed against the human enzyme. Large parts <strong>of</strong> this thesis were<br />
carried out at the <strong>University</strong> hospital under the guidance and supervision <strong>of</strong> Pr<strong>of</strong>. Pascke.<br />
International cooperations<br />
Maria Abramic, Ruder Boskovic <strong>Institute</strong> Zagreb, Croatia<br />
Steve Ealick, Cornell <strong>University</strong>, Ithaca, U.S.A.<br />
Toni Kutchan, Donald Danforth Plant Science Center, St. Louis, U.S.A.<br />
Shwu Liaw, National Yang-Ming <strong>University</strong>, Taipei, Taiwan<br />
Matthias Mack, Hochschule Mannheim, Germany<br />
Bruce Palfey, <strong>University</strong> <strong>of</strong> Michigan, Ann Arbor, U.S.A.<br />
Research projects<br />
FWF P22361: “Mechanism <strong>of</strong> redox controlled protein degradation”<br />
FWF P19858: “Enzymes <strong>of</strong> nikkomycin biosynthesis”<br />
FWF-Doktoratskolleg “Molecular Enzymology”<br />
WTZ <strong>Austria</strong>-Croatia “Structure-function relationships in metallopeptidases <strong>of</strong> the M49<br />
family”<br />
Publications<br />
1) Durchschein, K., Ferreira-da Silva, B., Wallner, S., Macheroux, P., Kroutil, W., Glueck,<br />
S. M., Faber, K.: The flavoprotein-catalyzed reduction <strong>of</strong> aliphatic nitro-compounds<br />
represents a biocatalytic equivalent to the Nef-reaction, Green Chemistry, 2010, 12:616-<br />
619.<br />
14
2) Jajcanin-Jozic, N., Deller, S., Pavkov, T., Macheroux, P., Abramic, M.: Identification <strong>of</strong><br />
the reactive cysteine residues in yeast dipeptidyl peptidase III, Biochimie, 2010, 92:89-<br />
96.<br />
Award<br />
Dissertation Award <strong>of</strong> the <strong>Austria</strong>n Society for Molecular Biosciences and Biotechnology<br />
(ÖGMBT) to Andreas Winkler for his dissertation on “Structure-function studies on<br />
berberine bridge enzyme (BBE) from the California poppy, Eschscholzia californica”. Dr.<br />
Andreas Winkler was a PhD student in the Doktoratskolleg “Molecular Enzymology” and is<br />
now a postdoctoral fellow at the Max-Planck <strong>Institute</strong> for Molecular Medicine in Heidelberg,<br />
Germany.<br />
15
Cell Biology Group<br />
Group leader: Günther Daum<br />
Postdoctoral Fellow: Karlheinz Grillitsch (since May 2010)<br />
PhD students: Melanie Connerth, Sona Rajakumari, Karlheinz Grillitsch (till March 2010),<br />
Miroslava Spanova, Susanne Horvath, Martina Gsell, Vid V. Flis, Vasyl’ Ivashov, Lisa<br />
Klug<br />
Master students: Brigitte Wagner, Gerald Mascher<br />
Technicians: Claudia Hrastnik, Alma Ljubijankic (since November 2010)<br />
General description<br />
Functional organelles are the basis for regulated processes within a cell. To sequester<br />
organelles from their environment, membranes are required which not only protect the interior<br />
<strong>of</strong> the organelles but also govern communication within the cell. To study biogenesis and<br />
maintenance <strong>of</strong> biological membranes and assembly <strong>of</strong> lipids into organelle membranes our<br />
laboratory makes use <strong>of</strong> the yeast as a well established experimental system. We combine<br />
biochemical, molecular and cell biological methods addressing problems <strong>of</strong> lipid metabolism,<br />
lipid depot formation and membrane biogenesis.<br />
Specific aspects studied recently in our laboratory are (i) assembly and homeostasis <strong>of</strong><br />
phosphatidylethanolamine in yeast organelle membranes with emphasis on the role <strong>of</strong> the<br />
major phosphatidylethanolamine synthesizing enzyme, the phosphatidylserine decarboxylase<br />
1, (ii) neutral lipid storage in lipid particles/droplets and mobilization <strong>of</strong> these depots with<br />
emphasis on the involvement <strong>of</strong> lipases and hydrolases, and (iii) characterization <strong>of</strong> organelle<br />
membranes from the industrial yeast Pichia pastoris.<br />
Phosphatidylethanolamine, a key component <strong>of</strong> yeast organelle membranes<br />
Work from our laboratory and from other groups had shown that phosphatidylethanolamine<br />
(PE), one <strong>of</strong> the major phospholipids <strong>of</strong> yeast membranes, is highly important for cellular<br />
function and cell proliferation. PE synthesis in the yeast is accomplished by a network <strong>of</strong><br />
reactions including (i) synthesis <strong>of</strong> phosphatidylserine (PS) in the endoplasmic reticulum,<br />
(ii) decarboxylation <strong>of</strong> PS by mitochondrial phosphatidylserine decarboxylase 1 (Psd1p) or<br />
(iii) Psd2p in a Golgi/vacuolar compartment, (iv) the CDP-ethanolamine pathway (Kennedy<br />
pathway) in the endoplasmic reticulum, and (v) the lysophospholipid acylation route catalyzed<br />
by Ale1p and Tgl3p. To obtain more insight into biosynthesis, assembly and homeostasis <strong>of</strong><br />
PE, single and multiple mutants bearing defects in the respective pathways can be used.<br />
Previous investigations in our laboratory were aimed at the molecular biological<br />
identification <strong>of</strong> novel components involved in PE homeostasis <strong>of</strong> the yeast Saccharomyces<br />
cerevisiae. For this purpose, a number <strong>of</strong> genetic screenings were performed. To obtain a<br />
global view <strong>of</strong> the role <strong>of</strong> PE in the cell and to study the effects <strong>of</strong> an unbalanced PE level we<br />
subjected a psd1∆ deletion mutant and the corresponding wild type to DNA microarray<br />
analysis and examined genome-wide changes in gene expression. Comparison <strong>of</strong> the gene<br />
expression pattern <strong>of</strong> the psd1∆ mutant with the wild type led to the identification <strong>of</strong> ~50<br />
differentially expressed genes. Grouping <strong>of</strong> these genes into functional categories revealed<br />
that PE formation by Psd1p influenced the expression <strong>of</strong> genes involved in diverse cellular<br />
pathways including transport, carbohydrate metabolism and stress response. Currently, the<br />
16
most promising candidates <strong>of</strong> this screening are under investigation. This study will provide<br />
novel evidence for the complex network <strong>of</strong> phospholipid synthesis in the yeast.<br />
To understand cellular PE homeostasis in more detail, we also performed experiments<br />
defining traffic routes <strong>of</strong> PE within the yeast cell. In recent studies, we investigated the<br />
plasma membrane as destination for PE traffic. We employed yeast mutants bearing defects<br />
in the different pathways <strong>of</strong> PE synthesis and demonstrated that PE formed through all four<br />
pathways can be supplied to the plasma membrane. The fatty acid composition <strong>of</strong> plasma<br />
membrane phospholipids was mostly influenced by the available pool <strong>of</strong> total species<br />
synthesized, although a certain balancing effect was observed regarding the assembly <strong>of</strong> PE<br />
species. We assume that the phospholipid composition <strong>of</strong> the plasma membrane is mainly<br />
affected by the synthesis <strong>of</strong> the respective components and subject to equilibrium, and to a<br />
lesser extent affected by specific transport and assembly processes.<br />
Psd1-precursor<br />
OM<br />
Tom<br />
40<br />
Tom<br />
IMS<br />
<br />
Psd1p-mature<br />
IM<br />
<br />
TIM23<br />
Oct<br />
1<br />
MP<br />
P<br />
Import <strong>of</strong> Psd1p into mitochondria<br />
A central aspect <strong>of</strong> this project is characterization <strong>of</strong> Psd1p regarding its molecular<br />
properties. Like most mitochondrial proteins, Psd1p is synthesized on free cytosolic<br />
ribosomes and imported into mitochondria where processing occurs. The Psd1-proenzyme<br />
contains a mitochondrial targeting sequence, an internal sorting sequence, and an alpha- and<br />
a beta-subunit which are linked through an LGST cleavage site. Cleavage at this site leads to<br />
the mature and active form <strong>of</strong> the enzyme generating a pyruvoyl group at the N-terminus <strong>of</strong><br />
the alpha subunit. In recent studies performed in collaboration with the laboratory <strong>of</strong> Pr<strong>of</strong>. N.<br />
Pfanner, Freiburg, Germany, we investigated i) the precise import route <strong>of</strong> Psd1p through the<br />
mitochondrial membranes, ii) the specific role <strong>of</strong> the LGST cleavage site on the import,<br />
assembly and maturation <strong>of</strong> the enzyme, (iii) the topology <strong>of</strong> Psd1p in the inner<br />
mitochondrial membrane; iv) the effect <strong>of</strong> mitochondrial processing peptidases on protein<br />
maturation, and v) possible complex formation <strong>of</strong> mature Psd1p.<br />
The link between PE metabolism and peroxisome proliferation is subject to another current<br />
investigation with emphasis on the role <strong>of</strong> enzymes and lipid transport routes involved.<br />
Previous studies suggested that PE formed through all four pathways (see above) and in<br />
different subcellular membranes can be supplied to peroxisomes with comparable efficiency.<br />
17
However, mechanisms involved in these translocation processes are still unclear. To address<br />
these questions, we established in vitro and in vivo assays for studying phospholipid supply<br />
to peroxisomal membranes. We constructed a strain which lacks the gene product <strong>of</strong> OPI3,<br />
the major PE methyltransferase localized to the ER, and bears an Opi3p-GFP hybrid with an<br />
SKL targeting sequence that directs the enzyme to peroxisomes. In this “reporter mutant”,<br />
the only site <strong>of</strong> phosphatidylcholine (PC) formation via methylation <strong>of</strong> PE are the<br />
peroxisomes, and the appearance <strong>of</strong> PC becomes an indicator and measure for PE<br />
translocation from the different sites <strong>of</strong> synthesis to peroxisomes. Currently permeabilized<br />
cells <strong>of</strong> the “reporter mutant” are used to characterize the PE transport to peroxisomes in<br />
some detail. This system in combination with mutations in the different PE biosynthetic<br />
pathways will allow us to investigate the different mechanisms <strong>of</strong> PE translocation between<br />
organelles involved in aminoglycerophospholipid biosynthesis.<br />
Most recently, we also studied the link between PE metabolism and neutral lipid storage. For<br />
this purpose we analyzed lipids from strains bearing defects in PE synthesis. These analyses<br />
showed that a mutant bearing a defect in the CDP-ethanolamine pathway had a decreased<br />
level <strong>of</strong> triacylglycerols (TAG). In cki1∆dpl1∆eki1∆ mutants bearing defects in the CDPethanolamine<br />
pathway both the cellular and the microsomal levels <strong>of</strong> PE were markedly<br />
decreased, whereas in other mutants <strong>of</strong> PE biosynthetic routes depletion <strong>of</strong> this<br />
aminoglycerophospholipid in microsomes was less pronounced. This observation is<br />
important because the TAG synthesisizing enzyme Lro1p similar to the enzymes <strong>of</strong> the CDPethanolamine<br />
pathway is a component <strong>of</strong> the ER. We conclude from these results that in<br />
cki1∆dpl1∆eki1∆ insufficient local supply <strong>of</strong> PE to Lro1p was a major reason for the strongly<br />
reduced TAG level.<br />
Neutral lipid storage in lipid particles and mobilization<br />
Yeast cells have the capacity to store neutral lipids TAG and STE (steryl esters) in subcellular<br />
structures named lipid particles/droplets. Upon requirement, TAG and STE can be mobilized<br />
and serve as building blocks for membrane biosynthesis. In a long-standing project <strong>of</strong> our<br />
laboratory, we investigate the characterization <strong>of</strong> enzymatic steps which lead to the<br />
mobilization <strong>of</strong> TAG and STE depots.<br />
A major focus <strong>of</strong> our neutral lipid project was the biochemical characterization <strong>of</strong> the three<br />
yeast TAG lipases, Tgl3p, Tgl4p and Tgl5p. Previous work from our laboratory had<br />
demonstrated that deletion <strong>of</strong> TGL3 encoding the major yeast TAG lipase resulted in<br />
decreased mobilization <strong>of</strong> TAG, a sporulation defect and a changed pattern <strong>of</strong> fatty acids,<br />
especially increased amounts <strong>of</strong> C22:0 and C26:0 very long chain fatty acids in the TAG<br />
fraction. To study a possible link between TAG lipolysis and membrane lipid biosynthesis, we<br />
carried out biochemical experiments with wild type and deletion strains bearing defects in<br />
Tgl3p, Tgl4p and Tgl5p. We demonstrated that tgl mutants had a lower level <strong>of</strong> sphingolipids<br />
and glycerophospholipids than wild type. ESI-MS/MS analyses confirmed that TAG<br />
accumulation in these mutant cells resulted in reduced amounts <strong>of</strong> phospholipids and<br />
sphingolipids. In vitro and in vivo experiments revealed that TAG lipolysis markedly affected<br />
the metabolic flux <strong>of</strong> long chain fatty acids and very long chain fatty acids required for<br />
sphingolipid and glycerophospholipid synthesis. The pattern <strong>of</strong> phosphatidylcholine (PC), PE<br />
and PS molecular species was altered in tgl deletion strains underlining the important role <strong>of</strong><br />
TAG turnover in maintenance <strong>of</strong> the pool size and remodelling <strong>of</strong> complex membrane lipids.<br />
This study shed new light on the physiological role <strong>of</strong> TAG lipases in yeast and in general.<br />
18
Even more surprising evidence was obtained when the enzymology <strong>of</strong> the three TAG lipases<br />
Tgl3p, Tgl4p and Tgl5p was studied in some detail. Motif search analysis indicated that Tgl3p<br />
and Tgl5p did not only contain the TAG lipase but also an acyltransferase motif. Interestingly,<br />
lipid analysis revealed that deletion <strong>of</strong> TGL3 resulted in a decrease and overexpression <strong>of</strong><br />
TGL3 in an increase <strong>of</strong> glycerophospholipids. Similar results were obtained with TGL5.<br />
Therefore, we tested purified Tgl3p and Tgl5p for acyltransferase activity. Indeed, both<br />
enzymes did not only exhibit lipase activity but also catalyzed acylation <strong>of</strong><br />
lysophosphatidylethanolamine and lysophosphatidic acid, respectively. Experiments using<br />
variants <strong>of</strong> Tgl3p created by site-directed mutagenesis clearly demonstrated that the two<br />
enzymatic activities act independently <strong>of</strong> each other. These results demonstrated that yeast<br />
Tgl3p and Tgl5p play a dual role in lipid metabolism contributing to both anabolic and<br />
catabolic processes.<br />
P-Lipase<br />
28 GGGTFG 29<br />
Acyltransferase<br />
48 HIIACQ 49<br />
1aa<br />
282 Patatin domain 48<br />
3<br />
910aa<br />
31 GSSAG 31<br />
Lipase<br />
Domains <strong>of</strong> Tgl3p (from Rajakumari and Daum, 2010, J. Biol. Chem. 285, 15769-15776)<br />
In another study, we demonstrated that the yeast TAG lipase Tgl4p, the functional ortholog <strong>of</strong><br />
adipose TAG lipase (ATGL), catalyzes multiple functions in lipid metabolism. An extended<br />
domain and motif search analysis revealed that Tgl4p bears not only a lipase consensus<br />
domain but also a conserved motif for calcium independent phospholipases A2 (PLA2). We<br />
showed that Tgl4p exhibits TAG lipase, STE hydrolase and PLA2 activities, but also<br />
catalyzes acyl-CoA dependent acylation <strong>of</strong> lysophosphatidic acid (LPA) to phosphatidic acid.<br />
Heterologous overexpression <strong>of</strong> Tgl4p in Pichia pastoris increased total phospholipid and<br />
specifically phosphatidic acid synthesis. Moreover, deletion <strong>of</strong> TGL4 in Saccharomyces<br />
cerevisiae showed an altered pattern <strong>of</strong> PC and phosphatidic acid molecular species.<br />
Altogether, our data suggested that yeast Tgl4p functions as hydrolytic enzyme in lipid<br />
degradation, but also contributes to fatty acid channelling and phospholipid remodelling.<br />
Several years ago we identified through a mass spectrometric approach for the first time the<br />
major lipid particle proteins <strong>of</strong> Saccharomyces cerevisiae. This approach was a milestone in<br />
the field because it identified a number <strong>of</strong> new gene products and their function and provided<br />
valuable hints for processes associated with lipid particles. Recently, a more precise lipid<br />
particle proteome analysis was initiated in collaboration with M. Karas from the <strong>Institute</strong> <strong>of</strong><br />
Pharmaceutical Chemistry, Johann Wolfgang Goethe <strong>University</strong>, Frankfurt, Germany. This<br />
proteome study was combined with a lipidomics investigation that was performed in<br />
collaboration with H. Köfeler from the Center for Medical Research, Medical <strong>University</strong> <strong>of</strong><br />
<strong>Graz</strong>, <strong>Austria</strong>. In this study, we compared lipid particle components from cells which were<br />
19
grown on glucose or on oleic acid. This approach identified a number <strong>of</strong> lipid particle proteins<br />
that were already known but also some novel polypeptide candidates. We also realized<br />
through this approach that there were some differences in the lipid particle proteome from<br />
cells grown on glucose or oleic acid. Finally, mass spectrometric analyses revealed marked<br />
differences in the lipidome <strong>of</strong> lipid particles from cells grown on the two different carbon<br />
sources. This study sets the stage for further investigation <strong>of</strong> protein-lipid interaction on the<br />
surface <strong>of</strong> lipid particles and provides basic evidence for the coordinated biosynthesis <strong>of</strong> lipid<br />
and protein components from lipid particles.<br />
Another important aspect <strong>of</strong> this project was to understand cell biological consequences <strong>of</strong><br />
dysfunctions in non-polar lipid storage. For this purpose, yeast cells were cultivated on oleic<br />
acid which was assumed to provoke lipotoxic stress. We found that under these cultivation<br />
conditions TAG synthesis was enhanced creating the major pool for the excess <strong>of</strong> fatty acids,<br />
whereas surprisingly STE synthesis was strongly inhibited. We showed that this effect was<br />
not due to decreased expression <strong>of</strong> ARE2 encoding the major yeast STE synthase at the<br />
transcriptional level, but to competitive enzymatic inhibition <strong>of</strong> Are2p by free oleic acid. As a<br />
result, a triple mutant dga1∆lro1∆are1∆ARE2 + grown on oleate did not form substantial<br />
amounts <strong>of</strong> STE and exhibited a growth phenotype similar to the dga1∆lro1∆are1∆are2∆<br />
quadruple mutant which lacks all four acyltransferases involved in neutral lipid synthesis and<br />
consequently also lacks lipid particles. Growth <strong>of</strong> these mutants on oleate was strongly<br />
delayed and cell viability was decreased, but rescued by an adaptation process. In these<br />
strains, oleate stress caused morphological changes <strong>of</strong> intracellular membranes, altered<br />
phospholipid composition and increased formation <strong>of</strong> ethyl esters as a possible buffer for fatty<br />
acids.<br />
Another potential non-polar storage lipid is squalene. This component belongs to the group <strong>of</strong><br />
isoprenoids and is precursor for the synthesis <strong>of</strong> sterols, steroids and ubiquinons. In a previous<br />
study we had demonstrated that squalene accumulates in yeast strains bearing a deletion <strong>of</strong> the<br />
HEM1 gene. In such strains, the vast majority <strong>of</strong> squalene is stored in lipid particles/droplets<br />
together with TAG and STE. In mutants lacking the ability to form lipid particles, however,<br />
substantial amounts <strong>of</strong> squalene accumulate in organelle membranes. In a recent study, we<br />
investigated the effect <strong>of</strong> squalene on biophysical properties <strong>of</strong> lipid particles and membranes<br />
and compared these results to artificial membranes. Our experiments showed that squalene<br />
lowered the order <strong>of</strong> STE shells in lipid particles. The majority <strong>of</strong> squalene, however, was<br />
localized to the center <strong>of</strong> lipid particles where it formed a s<strong>of</strong>t core together with TAG. This<br />
view was confirmed with model lipid particles. In biological and artificial membranes<br />
fluorescence spectroscopy studies revealed that it is not the absolute squalene level per se, but<br />
the squalene to ergosterol ratio which mainly affects membrane fluidity/rigidity. In a fluid<br />
membrane environment squalene induces rigidity <strong>of</strong> the membrane, whereas in rigid<br />
membrane there is almost no additive effect <strong>of</strong> squalene. Our results demonstrated that<br />
squalene (i) can be well accommodated in yeast lipid particles and organelle membranes<br />
without causing deleterious effects; and (ii) although not being a typical membrane lipid may<br />
be regarded as a mild modulator <strong>of</strong> biophysical membrane properties.<br />
Pichia pastoris organelles and lipids<br />
The yeast Pichia pastoris is an important experimental system for heterologous expression <strong>of</strong><br />
proteins. Nevertheless, surprisingly little is known about organelles <strong>of</strong> this microorganism.<br />
For this reason, we started a systematic biochemical and cell biological study to establish<br />
standardized methods <strong>of</strong> Pichia pastoris organelle isolation and characterization. Recent work<br />
20
focused on the biochemical characterization <strong>of</strong> the plasma membrane and secretory organelles<br />
from Pichia pastoris. One major aim <strong>of</strong> this project is the qualitative and quantitative analysis<br />
<strong>of</strong> phospholipids, fatty acids and sterols from organelle membranes when Pichia pastoris is<br />
grown on different carbon sources. For this purpose, methods for the isolation <strong>of</strong> Pichia<br />
pastoris organelle fractions were established. Standardized techniques <strong>of</strong> lipid analysis<br />
including lipidome analyses are currently employed to address these problems.<br />
Doctoral Theses completed<br />
Sona Rajakumari: Role <strong>of</strong> yeast triacylglycerol lipases in membrane lipid metabolism<br />
Previous work from our laboratory had demonstrated that gene products <strong>of</strong> TGL3, TGL4 and<br />
TGL5 encoding the major yeast triacylglycerol (TAG) lipases are located to lipid particles.<br />
Deletion <strong>of</strong> TGL3 and TGL4 resulted in a decreased mobilization <strong>of</strong> TAG from the lipid<br />
particles and a sporulation defect. TAG stored in tgl3∆ and tgl5∆ deletion strains contains<br />
slightly increased amounts <strong>of</strong> C22:0 and C26:0 very long chain fatty acids (VLCFAs)<br />
compared to wild type. These VLCFAs are indispensable for sphingolipid biosynthesis and<br />
crucial for raft association in yeast. Moreover, deletion <strong>of</strong> these TAG lipases results in a<br />
decreased level <strong>of</strong> membrane lipid biosynthesis. In this Thesis the role <strong>of</strong> TAG lipases in<br />
membrane lipid metabolism <strong>of</strong> the yeast Saccharomyces cerevisiae was studied in some<br />
detail. First, a metabolic link between TAG lipolysis by TAG lipases and sphingolipid and<br />
phospholipid synthesis was shown. Secondly, a dual function <strong>of</strong> Tgl3p and Tgl5p as lipases<br />
and as lysophosphatidylethanolamine or lysophosphatidic acid acyltransferase, respectively,<br />
was demonstrated. We also showed that the acyltransferase but not the lipase function <strong>of</strong><br />
Tgl3p was essential for efficient sporulation. Finally, we also characterized Tgl4p as<br />
multifunctional protein exhibiting lipase, phospholipase A2, acyl-CoA dependent LPA<br />
acyltransferase and STE hydrolase activity. Altogether, this work shows that catabolic and<br />
anabolic activities <strong>of</strong> TAG lipases play a pivotal role in maintaining lipid homeostasis in the<br />
yeast.<br />
Karlheinz Grillitsch: Lipid storage and mobilization in the yeast Saccharomyces cerevisiae<br />
The yeast Saccharomyces cerevisiae like higher eukaryotic cells (mammals and plants) and<br />
Gram-positive bacteria contains a specified organelle for lipid storage, the lipid particle (LP).<br />
Unlike other organelles, LP are covered by a phospholipid monolayer that protects its<br />
hydrophobic interior formed from densely packed non-polar lipids steryl esters (STE) and<br />
triacylglycerols (TAG). Moreover, LP contain a small but specific set <strong>of</strong> proteins. In this<br />
Thesis, storage and mobilization <strong>of</strong> yeast neutral lipids were studied. First, biochemical<br />
properties <strong>of</strong> the three STE hydolases, Tgl1p, Yeh1p and Yeh2p were investigated. Analysis<br />
<strong>of</strong> enzymatic properties revealed distinct substrate specificities <strong>of</strong> the three proteins and<br />
involvement in sterol homeostasis. Sterol homeostasis is also linked to cell polarity. We<br />
showed that two effectors <strong>of</strong> cell polarity, Ste20p and Cla4p, function as negative modulators<br />
<strong>of</strong> sterol biosynthesis. A major part <strong>of</strong> this Thesis was devoted to description <strong>of</strong> the molecular<br />
composition <strong>of</strong> the yeast LP and its modulation upon changes in cultivation conditions. For<br />
this purpose, LP from cells grown on either glucose or oleate were analyzed. Strong<br />
incorporation <strong>of</strong> the mono-unsaturated oleic acid into TAG and most phospholipids was<br />
observed upon shifting cells to oleate medium. Most notably, the balanced 1:1 ratio <strong>of</strong> TAG to<br />
STE in cells grown on glucose was strongly increased on oleate. Change <strong>of</strong> the medium also<br />
led to changes in the LP protein pattern. This was demonstrated by a combined<br />
21
lipidomic/proteomic approach which also revealed several novel putative LP proteins. Finally,<br />
LP protein targeting and topology were studied using the squalene epoxidase Erg1p, a typical<br />
LP protein, as a model. These studies showed that the majority <strong>of</strong> the protein faced the<br />
cytosol, and only a small part was protected by the membrane. We assume that this may be a<br />
general feature <strong>of</strong> LP proteins.<br />
Melanie Connerth: Lipid traffic in the yeast<br />
The yeast Saccharomyces cerevisiae is a suitable organism for studies <strong>of</strong> lipid homeostasis in<br />
the cell. Processes involved in the biosynthesis <strong>of</strong> lipid compounds such as fatty acids, neutral<br />
lipids and phospholipids are well understood and located to different subcellular<br />
compartments. Two major yeast organelles involved in fatty acid turnover are peroxisomes<br />
harboring steps <strong>of</strong> beta-oxidation and lipid particles (LP) which are the storage organelle for<br />
excess fatty acids in the cell. Biogenesis <strong>of</strong> both compartments is not completely understood<br />
yet and little is known about mechanisms <strong>of</strong> lipid supply to either <strong>of</strong> these organelles. This<br />
Thesis was aimed at elucidation <strong>of</strong> lipid traffic routes towards and from peroxisomes and LP<br />
and the interplay <strong>of</strong> fatty acid utilization processes in both compartments. Initially,<br />
phospholipid supply to peroxisomal membranes was investigated in some detail with focus on<br />
phosphatidylethanolamine (PE) transport to peroxisomes. These studies revealed that the four<br />
different PE biosynthetic pathways contributed with different efficiency to this process.<br />
Moreover, a hybrid <strong>of</strong> the PE methyltransferase Opi3p was introduced into peroxisomes <strong>of</strong> an<br />
OPI3 deletion mutant which allowed us to measure transport <strong>of</strong> PE to peroxisomes by<br />
appearance <strong>of</strong> phosphatidylcholine (PC) in peroxisomal membranes. The major take-home<br />
message from these experiments was that direct membrane contact most likely accounts for<br />
delivery <strong>of</strong> lipids from their sites <strong>of</strong> synthesis to peroxisomes. During these studies, a link <strong>of</strong><br />
peroxisomes with LP, the neutral lipid storage compartment, was also discovered. It was<br />
shown that exogenous oleic acid was primarily stored in LP in the form <strong>of</strong> triacylglycerols<br />
(TAG) and subsequently mobilized by a subset <strong>of</strong> already known as well as novel<br />
lipases/hydrolases for supply to peroxisomes. In the course <strong>of</strong> complete lipidome and<br />
proteome analyses <strong>of</strong> LP a novel and specific inhibitory effect <strong>of</strong> oleate on the activity <strong>of</strong> the<br />
steryl ester synthase Are2p was discovered thus revealing a new aspect <strong>of</strong> yeast lipotoxicity.<br />
In summary, data from this Thesis showed that distinct pools <strong>of</strong> fatty acids serving for<br />
different cellular processes exist in the cell, and LP play a major role in regulating lipid<br />
homeostasis. These data are a step forward in our understanding <strong>of</strong> cellular lipid traffic which<br />
may also be applicable to higher eukaryotic cells such as plants and mammals.<br />
International cooperations<br />
N. Pfanner, <strong>Institute</strong> <strong>of</strong> <strong>Biochemistry</strong> and Molecular Biology, ZBMZ, <strong>University</strong> <strong>of</strong> Freiburg,<br />
Germany<br />
I. Hapala, Slovak Academy <strong>of</strong> Sciences, <strong>Institute</strong> <strong>of</strong> Animal <strong>Biochemistry</strong> and Genetics,<br />
Ivanka pri Dunaji, Slovak Republic<br />
I. Feussner, Plant <strong>Biochemistry</strong>, Albrecht-von-Haller-<strong>Institute</strong> <strong>of</strong> Plant Sciences, Georg-<br />
August <strong>University</strong> Göttingen, Germany<br />
R. Erdmann, <strong>Institute</strong> <strong>of</strong> Physiological Chemistry, Ruhr-<strong>University</strong> Bochum, Germany<br />
M. Karas, <strong>Institute</strong> <strong>of</strong> Pharmaceutical Chemistry, Johann Wolfgang Goethe <strong>University</strong>,<br />
Frankfurt, Germany<br />
R. Rajasekharan, Department <strong>of</strong> <strong>Biochemistry</strong>, Indian <strong>Institute</strong> <strong>of</strong> Science, Bangalore, India<br />
22
Research projects<br />
FWF P21429: Phosphatidylserine decarboxylase<br />
FWF P18857: Neutral lipid storage and mobilization in the yeast Saccharomyces cerevisiae<br />
FWF L-178 (Translational Research): Characterization <strong>of</strong> organelles from the yeast Pichia<br />
pastoris<br />
FWF PhD Program: Molecular Enzymology<br />
FWF P23029 Lipases <strong>of</strong> the yeast Saccharomyces cerevisiae<br />
<strong>Austria</strong>n Center <strong>of</strong> Industrial Biotechnology (ACIB): Pichia pastoris Cell factory and Protein<br />
Production<br />
Invited Lecture<br />
1. S. Rajakumari, M. Connerth, K. Grillitsch, S. Horvath, R. Rajasekharan and G. Daum<br />
Fatty acid channeling from depots to biomembranes in the yeast<br />
International Lipid Symposium: Cell Biology and Metabolism, Beijing, China, 1-3<br />
September 2010<br />
Publications<br />
1. Rajakumari, S. and Daum, G.<br />
Janus-faced enzymes yeast Tgl3p and Tgl5p catalyze lipase and acyltransferase<br />
reactions<br />
Mol. Biol. Cell 21 (2010) 501-510<br />
2. Schuiki, I., Schnabl, M., Czabany, T., Hrastnik, C. and Daum, G.<br />
Phosphatidylethanolamine synthesized by four different pathways is supplied to the<br />
plasma membrane <strong>of</strong> the yeast Saccharomyces cerevisiae.<br />
Biochim. Biophys. Acta 1801 (2010) 480-486<br />
3. Spanova, M., Czabany, T., Zellnig, G., Leitner, E., Hapala, I. and Daum, G.<br />
Effect <strong>of</strong> lipid particle biogenesis on the subcellular distribution <strong>of</strong> squalene in the yeast<br />
Saccharomyces cerevisiae.<br />
J. Biol. Chem. 285 (2010) 6127-6133<br />
4. Rajakumari, S. and Daum, G.<br />
Multiple functions as lipase, steryl ester hydrolase, phospholipase and acyltransferase <strong>of</strong><br />
Tgl4p from the yeast Saccharomyces cerevisiae.<br />
J. Biol. Chem. 285 (2010) 15769-15776<br />
5. Wagner, M., Hoppe, K., Czabany, T., Heilmann, M., Daum, G., Feussner, I. and Fulda,<br />
M.<br />
Identification and characterization <strong>of</strong> an acyl-CoA:diacylglycerol acyltransferase 2<br />
(DGAT2) gene from the microalga O. tauri.<br />
Plant Physiol. Biochem. 48 (2010) 407-416<br />
6. Connerth, M., Czabany, T., Wagner, A., Zellnig, G., Leitner, E., Steyrer, E. and Daum,<br />
G.<br />
Oleate inhibits steryl ester synthesis and causes liposensitivity in the yeast.<br />
J. Biol. Chem. 285 (2010) 26832–26841<br />
7. Rajakumari, S, Rajasekharan, R. and Daum, G.<br />
Triacylglycerol lipolysis is linked to sphingolipid and phospholipid metabolism <strong>of</strong> the<br />
yeast Saccharomyces cerevisiae.<br />
Biochim. Biophys. Acta 1801 (2010) 1314-1322<br />
23
Molecular Biology Group<br />
Group leader: Karin Athenstaedt<br />
Postdoctoral Fellow: Andreas Beranek<br />
Acyltransferases catalyzing triacylglycerol synthesis in the oleaginous yeast Yarrowia<br />
lipolytica<br />
The oleaginous yeast Yarrowia lipolytica has an outstanding capacity to accumulate huge<br />
amounts <strong>of</strong> triacylglycerols (TAG). Along with other neutral lipids, TAG are stored in socalled<br />
lipid particles, cell compartments with a rather simple structure. A neutral lipid core<br />
mainly formed <strong>of</strong> TAG and steryl esters is surrounded by a phospholipid monolayer with few<br />
proteins embedded. By growing Yarrowia lipolytica cells in media containing, e.g., industrial<br />
fats or glycerol as a carbon source, the amount <strong>of</strong> TAG can be increased up to 40% <strong>of</strong> cell dry<br />
weight. This ability leads to its application in biotechnological processes such as single cell<br />
oil production or production <strong>of</strong> nutrients enriched in essential fatty acids which can serve as<br />
nutritional complements. However, Yarrowia lipolytica may also serve as a model organism<br />
to study lipid turnover in adipocytes, since not only the ability to store excessive amounts <strong>of</strong><br />
TAG in lipid particles but also the composition <strong>of</strong> this cell compartment resembles adipocytes<br />
<strong>of</strong> higher eukaryotes. Despite these potentials <strong>of</strong> Yarrowia lipolytica information about TAG<br />
(lipid) metabolism in this yeast is rather limited. Thus, we started to investigate the proteome<br />
<strong>of</strong> Yarrowia lipolytica for proteins involved in TAG synthesis. Homology searches with TAG<br />
synthases <strong>of</strong> other eukaryotes as queries highlighted two candidate gene-products <strong>of</strong> the<br />
oleaginous yeast potentially catalyzing the formation <strong>of</strong> TAG. A decreased amount <strong>of</strong> TAG in<br />
mutant cells defective in these candidate genes already pinpointed to a function <strong>of</strong> these<br />
polypeptides in TAG formation. To investigate whether these candidate genes encode true<br />
TAG synthases these genes were heterologously expressed in cells <strong>of</strong> a Saccharomyces<br />
cerevisiae mutant defective in neutral lipid synthesis and as a consequence lacking lipid<br />
particles (Fig. 1).<br />
A B<br />
C D<br />
Figure 1: Restoration <strong>of</strong> lipid particle formation upon heterologous expression <strong>of</strong> Yarrowia<br />
lipolytica TAG synthase candidates in mutant cells <strong>of</strong> the budding yeast Saccharomyces<br />
cerevisiae lacking lipid particles.<br />
In contrast to the negative control (B; mutant + empty plasmid), lipid particles are formed in<br />
mutant cells transformed with plasmids bearing either <strong>of</strong> the respective candidate genes <strong>of</strong><br />
Yarrowia lipolytica (C, D). Panel A shows lipid particle formation in a wild-type cell <strong>of</strong><br />
Saccharomyces cerevisiae. Lipid particles are indicated by arrows. Size bar: 10 µm.<br />
Fluorescent microscopic inspection and lipid analyses <strong>of</strong> the respective mutants clearly<br />
demonstrated that these Yarrowia lipolytica genes encode true TAG synthases. The<br />
24
characteristics <strong>of</strong> these two TAG synthases <strong>of</strong> the oleaginous yeast are currently under<br />
investigation.<br />
Biosynthesis <strong>of</strong> phosphatidic acid in yeast<br />
Phosphatidic acid is <strong>of</strong> utmost importance, since it is the key intermediate for the formation <strong>of</strong><br />
all glycerolipids, namely glycerophospholipids (membrane lipids) and triacylglycerols<br />
(storage lipids). Furthermore, this lipid functions in cell signaling. In all eukaryotic organisms<br />
enzymes catalyzing phosphatidic acid biosynthesis occur in redundancy. One reason for the<br />
existence <strong>of</strong> isoenzymes may be the formation <strong>of</strong> different phosphatidic acid pools supplying<br />
different pathways. In this project this hypothesis is tested in the model organism yeast<br />
Saccharomyces cerevisiae, which contains two glycerol-3-phosphate acyltransferases, Gat1p<br />
and Gat2p, and two 1-acyl glycerol-3-phosphate acyltransferases, Slc1p and Lpt1p. Glycerol-<br />
3-phosphate acyltransferases catalyze the first and rate limiting step in de novo synthesis <strong>of</strong><br />
phosphatidic acid via the glycerol-3-phosphate pathway, namely the acylation <strong>of</strong> glycerol-3-<br />
phosphate yielding 1-acyl glycerol-3-phosphate (lyso-phosphatidic acid). A subsequent<br />
acylation converts lyso-phosphatidic acid to phosphatidic acid and is catalyzed by a 1-acyl<br />
glycerol-3-phosphate acyltransferase.<br />
The precise localization <strong>of</strong> glycerol-3-phosphate acyltransferases tagged with green<br />
fluorescent protein (GFP) has been determined by fluorescence microscopy and Western blot<br />
analyses with highly purified cell fractions <strong>of</strong> the respective mutants. Whereas Gat1p is<br />
associated with lipid particles and the endoplasmic reticulum, Gat2p is exclusively localized<br />
to the latter compartment in wild-type background (Fig. 2).<br />
A<br />
B<br />
LP<br />
LP<br />
Wild-type + GFP-Gat1p<br />
Wild-type + GFP-Gat2p<br />
Figure 2: Fluorescence microscopy <strong>of</strong> Gat1p<br />
and Gat2p, respectively, tagged with green<br />
fluorescent protein (GFP) in wild-type<br />
background.<br />
A: GFP-Gat1p localizes to the endoplasmic<br />
reticulum and lipid particles (indicated by<br />
arrows). In contrast, GFP-Gat2p is only present<br />
in the endoplasmic reticulum (B).<br />
Most interestingly, the absence <strong>of</strong> either glycerol-3-phosphate acyltransferase results in a<br />
different distribution pattern <strong>of</strong> its counterpart, and in growth defects. Whether these<br />
phenotypes are caused by alterations in the lipid pattern <strong>of</strong> the respective mutants is currently<br />
under investigation.<br />
International cooperations<br />
T. Chardot, Institut National de la Recherche Agronomique, Institut Jean-Pierre Bourgin,<br />
UMR1318, Versailles, France<br />
J.-M. Nicaud, Institut National de la Recherche Agronomique, Laboratoire de Microbiologie<br />
et Génétique Moléculaire, UMR1319, Jouy-en-Josas, France<br />
25
Research projects<br />
FWF P21251: Biosynthesis <strong>of</strong> phosphatidic acid in yeast<br />
Publications<br />
1) Athenstaedt, K.:<br />
Neutral lipids in yeast: synthesis, storage and degradation.<br />
In Microbiology <strong>of</strong> Hydrocarbons, Oils, Lipids, and Derived Compounds. (Ed. by K. N.<br />
Timmis) Springer-Heidelberg, 2010, 471-480.<br />
2) Athenstaedt, K.:<br />
Players in the neutral lipid game – proteins involved in neutral lipid metabolism in<br />
yeast.<br />
In Microbiology <strong>of</strong> Hydrocarbons, Oils, Lipids, and Derived Compounds. (Ed. by K. N.<br />
Timmis) Springer-Heidelberg, 2010, 537-546.<br />
3) Athenstaedt, K.:<br />
Isolation and characterization <strong>of</strong> lipid particles <strong>of</strong> yeast.<br />
In Microbiology <strong>of</strong> Hydrocarbons, Oils, Lipids, and Derived Compounds. (Ed. by K. N.<br />
Timmis) Springer-Heidelberg, 2010, 4223-4229.<br />
4) Athenstaedt, K.:<br />
Neutral lipid metabolism in yeast as a template for biomedical research.<br />
In Microbiology <strong>of</strong> Hydrocarbons, Oils, Lipids, and Derived Compounds. (Ed. by K. N.<br />
Timmis) Springer-Heidelberg, 2010, 3381-3382.<br />
26
Biophysical chemistry group<br />
Group leader: Albin Hermetter<br />
Postdoctoral Fellow: Heidemarie Ehammer<br />
PhD students: Ute Stemmer, Daniel Koller, Claudia Ramprecht, Lingaraju Marlingapla<br />
Halasiddappa, Bojana Stojcic<br />
Master students: Andreas Gasser, Gabriel Pürstinger, Hannah Jaritz<br />
Technicians: Elfriede Zenzmaier<br />
General description<br />
Our research deals with the role <strong>of</strong> glycero(phospho)lipids and lipid modifying enzymes as<br />
components <strong>of</strong> membranes and lipoproteins, their function as mediators in cellular<br />
(patho)biochemistry, and their application as analytical tools in enzyme technology.<br />
Fluorescence spectroscopy is used as a main technique to investigate the behaviour <strong>of</strong> these<br />
biomolecules in the respective supramolecular systems.<br />
Section 1 <strong>of</strong> the following report summarizes our studies on lipid oxidation, the effects <strong>of</strong><br />
oxidized lipids on intracellular signalling, and the inhibition <strong>of</strong> these processes by synthetic<br />
and natural antioxidants.<br />
Section 2 describes the development <strong>of</strong> fluorescence for functional proteomic analysis <strong>of</strong><br />
lipolytic enzymes in microbial, animal and human cells.<br />
1. Lipid oxidation and atherosclerosis – Lipotoxicity<br />
1.1. Interaction <strong>of</strong> oxidized phospholipids with vascular cells<br />
Sphingomyelin<br />
acid<br />
Sphingomyelinase<br />
Oxidized -<br />
Phospholipids<br />
O<br />
H<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O P O<br />
OH<br />
O<br />
O<br />
O<br />
O P O<br />
OH<br />
+<br />
N<br />
N +<br />
Ceramide<br />
OXPHOS-EuroMEMBRANE<br />
JNK<br />
p38 MAPK<br />
Caspase 3<br />
ERK<br />
AKT/PKB<br />
NFkB<br />
PROLIFERATION<br />
SURVIVAL<br />
Interactions <strong>of</strong> oxidized lipoproteins with<br />
the cells <strong>of</strong> the arterial wall induce and<br />
influence the progress <strong>of</strong> atherosclerosis.<br />
Accumulation <strong>of</strong> foam cells originating<br />
from macrophages and excessive intimal<br />
growth <strong>of</strong> vascular smooth muscle cells<br />
(SMC) alternating with focal massive cell<br />
death are characteristics typical <strong>of</strong> the atherosclerotic lesion. These phenomena are largely<br />
mediated by the oxidized phospholipid components <strong>of</strong> the modified particles that are<br />
generated under the conditions <strong>of</strong> oxidative stress. In the framework <strong>of</strong> the research consortia<br />
LIPOTOX (SFB) and OXPHOS (EuroMEMBRANE), we investigate the “Toxicity <strong>of</strong><br />
oxidized phospholipids in macrophages” and “The protein targets and apoptotic signaling <strong>of</strong><br />
oxidized phospholipids”. These studies aim at identifying the molecular and cellular effects <strong>of</strong><br />
an important subfamily <strong>of</strong> the oxidized phospholipids containing long hydrocarbon chains in<br />
27
position sn-1 and short polar acyl residues in position sn-2 <strong>of</strong> the glycerol backbone. The<br />
respective compounds trigger an intracellular signaling network including sphingomyelinases,<br />
(MAP) kinases and transcription factors thereby inducing proliferation or apoptosis <strong>of</strong><br />
vascular cells. Both phenomena largely depend on the specific action <strong>of</strong> sphingolipid<br />
mediators that are acutely formed upon cell stimulation by the oxidized lipids. Fluorescence<br />
microscopic studies on labeled lipid analogs revealed that the short-chain<br />
phosphatidylcholines are easily transferred from the aqueous phase into the cell plasma<br />
membrane and eventually spread throughout the cells. Under these circumstances, the biologically<br />
active compounds are very likely to interfere<br />
directly with various signaling components inside<br />
the cells, which finally decide about cell growth or<br />
death. Investigations are being performed on the<br />
levels <strong>of</strong> the lipidome, the apparent enzyme<br />
activities, the proteome an the transcriptome to find<br />
the primary molecular targets and their downstream<br />
elements that are the key compnents <strong>of</strong> lipid-induced<br />
cell death.<br />
1.2. Oxidative stress and antioxidants<br />
Antioxidants protect biomolecules against<br />
oxidative stress and thus, may prevent its<br />
pathological consequences. Specific fluorescent<br />
markers have been established for high-throughput<br />
screening <strong>of</strong> lipid and protein oxidation and its<br />
inhibition by natural and synthetic antioxidants.<br />
These methods can also be used for the<br />
determination <strong>of</strong> antioxidant capacities <strong>of</strong><br />
biological fluids (e.g. serum) and edible oils. A<br />
prominent example is pumpkin seed oil which<br />
contains a variety <strong>of</strong> antioxidants (e.g. tocopherols<br />
and phenolic substances) and other secondary plant<br />
components that possess useful biological<br />
properties.<br />
2. Functional proteomic analysis <strong>of</strong> lipolytic enzymes<br />
The functional properties <strong>of</strong> lipases and phospholipases are the subject <strong>of</strong> our studies in the<br />
framework <strong>of</strong> the joint research program GOLD-Genomics <strong>of</strong> Lipid-associated Disorders at<br />
KFU <strong>Graz</strong>. Lipolytic enzymes catalyze intra- and extracellular lipid degradation.<br />
Dysfunctions in lipid metabolism may lead to various diseases including obesity, diabetes or<br />
atherosclerosis. In chemistry, lipases and esterases are important biocatalysts for<br />
(stereo)selective reactions on synthetic and natural substrates leading to defined products for<br />
pharmaceutical or agrochemical use.<br />
28
2.1. Fluorescent suicide inhibitors for in-gel analysis <strong>of</strong> lipases and phospholipases<br />
Fluorescent suicide inhibitors have been developed as activity recognition probes (ARPs) for<br />
qualitative and quantitative analysis <strong>of</strong> active lipolytic enzymes in complex biological<br />
samples (industrial enzyme preparations, serum, cells, tissues). Since inhibitor binding to the<br />
active sites <strong>of</strong> lipases and esterases is specific and stoichiometric, accurate information can be<br />
obtained about the type <strong>of</strong> enzyme and the moles <strong>of</strong> active protein (active sites) in<br />
electrophoretically pure or heterogeneous enzyme preparations.<br />
Labelling <strong>of</strong> enzymes with an ARP specific for serine hydrolases<br />
BG<br />
Inactive<br />
Inactive<br />
RG NBD<br />
RG: Reacti v e p h osphonate gr o up,<br />
irreversibly inhibits the catalytic serine<br />
Active<br />
BG<br />
BG: Binding group: is specifically recognized<br />
by the active enzyme<br />
Inactive<br />
Inactive<br />
Inhibited<br />
RG<br />
NBD<br />
NBD : fluorescent tag<br />
λ : 488 nm; λ : 540 nm<br />
ex<br />
em<br />
Fluorescent inhibitors are currently applied to proteomic analysis <strong>of</strong> the lipolytic enzymes in<br />
human and animal cells. These studies are performed in the framework <strong>of</strong> the joint project<br />
GOLD (Genomics <strong>of</strong> Lipid-associated Disorders/ coordinated by KFU <strong>Graz</strong>) which aims at<br />
discovering novel genes, processes and pathways that regulate lipid homeostasis in humans,<br />
mice and yeast. This is one <strong>of</strong> the projects in the field <strong>of</strong> functional genomics (GEN-AU,<br />
GENome research in AUstria) funded by the <strong>Austria</strong>n Federal Ministry for Education,<br />
Science and Culture (bm:bwk).<br />
Green: wt Red: ko Red: wt Green: ko<br />
DABGE Analysis <strong>of</strong><br />
lipases and esterases<br />
in mouse adipose tissue<br />
<strong>of</strong> wt and lipase ko<br />
mice<br />
Differential activity-based gel electrophoresis (DABGE) was developed for comparative<br />
analysis <strong>of</strong> two lipolytic proteomes in one polyacrylamide gel. For this purpose, the active<br />
lipases/esterases <strong>of</strong> two different samples are labelled with fluorescent inhibitors that possess<br />
identical substrate analogous structures but carry different cyanine dyes as reporter<br />
fluorophores. After sample mixing and protein separation by 1-D or 2-D PAGE, the enzymes<br />
carrying the sample-specific colors are detected and quantified. This technique can be used<br />
29
for the determination <strong>of</strong> differences in enzyme patterns, e.g. due to effects <strong>of</strong> genetic<br />
background, environment, metabolic state and disease.<br />
Master Theses completed:<br />
Andreas Gasser: Determination <strong>of</strong> substrate- and stereoselectivity <strong>of</strong> lipases<br />
The elucidation <strong>of</strong> the reaction mechanisms <strong>of</strong> lipolytic enzymes requires the determination <strong>of</strong><br />
the substrate- and stereoselectivities <strong>of</strong> these proteins. In order to find a more straightforward<br />
alternative to the common assays using radioactive substrates, a method using chiral, pyrenelabelled<br />
substrates was developed in this study, which allows the determination <strong>of</strong> substrateand<br />
stereoselectivity <strong>of</strong> lipases. This technique avoids the application <strong>of</strong> radioactive isotopes<br />
and as a consequence time consuming procedures. It is the basis for a simple and fast<br />
determination <strong>of</strong> lipase activity in high throughput analysis. On the basis <strong>of</strong> the robust and<br />
well characterized fungal Rhizomucor miehei lipase, the fluorescent substrates were tested for<br />
their applicability to determine substrate- and stereoselectivity <strong>of</strong> lipolytic enzymes using<br />
simple thin-layer chromatography. In order to obtain quantitative results and higher<br />
throughput, the method was adapted to HPLC analysis. This version was used to analyze<br />
activities as well as e.e. values <strong>of</strong> Rhizomucor miehei lipase, Chromobacterium viscosum<br />
lipase and Candida cylindracea cholesterol esterase. After overexpression in COS-7 cells, the<br />
selectivity and activity <strong>of</strong> the animal lipases adipose triglyceride lipase (ATGL), hormone<br />
sensitive lipase (HSL) and monoglyceride lipase (MGL) were measured in total cell lysates.<br />
Lipolytic activities were also determined in adipose tissue homogenates <strong>of</strong> ATGL- and HSLdeficient<br />
mice and compared to the wild type animals. The deficiency in the respective lipases<br />
correlated with the degradation patterns <strong>of</strong> the fluorescent substrates as determined by the<br />
above described method.<br />
Franziska Vogl: Transfection <strong>of</strong> macrophages with siRNA and plasmids. Expression and<br />
silencing <strong>of</strong> proteins targeted by oxidized phospholipids<br />
Oxidized phospholipids (oxPL) are generated from (poly)unsaturated glycerophospholipids in<br />
membranes and lipoproteins under conditions <strong>of</strong> oxidative stress. These compounds induce<br />
apoptosis in the cells <strong>of</strong> the vascular wall. In a previous functional proteomics study, the<br />
primary protein targets <strong>of</strong> a toxic aldehydophospholipid were identified in macrophages. To<br />
find out which <strong>of</strong> these protein candidates are involved in lipid-mediated cell death, we are<br />
following two different strategies. The first one uses siRNA technology to knock down the<br />
target proteins and measure the effect on cell death and signalling. The second approach is<br />
based on fusion proteins containing the protein candidates and RFP to measure Försterresonance-energy-transfer<br />
(FRET) and determine the spatial proximity to fluorescently<br />
labelled oxPL. In this diploma thesis, protocols for chemical transfection <strong>of</strong> RAW 264.7 cells<br />
with expression plasmids were optimized. Five fluorescent fusion proteins were cloned and<br />
expressed in Cos-7 cells. Chemical transfection and electroporation were used to transfect<br />
cells with siRNA against GAPDH and Caspase-3. Transfections and silencing were monitored<br />
using FACS-measurements, Western blotting and qPCR and proved to be efficient in both<br />
cases. Silencing <strong>of</strong> caspase-3 was associated with a decrease in protein expression and loss <strong>of</strong><br />
function. As a consequence, enzyme knock-down rendered the cells more resistant towards<br />
oxPL-induced apoptosis, supporting the assumption that the protease is causally involved in<br />
programmed cell death.<br />
30
International cooperations:<br />
T. Hianik, Department <strong>of</strong> Biophysics and Chemical Physics, Comenius <strong>University</strong>, Bratislava,<br />
Slovakia<br />
T. Hornemann, <strong>Institute</strong> for Clinical Chemistry, <strong>University</strong> Hospital Zürich; Zürich,<br />
Switzerland<br />
M. H<strong>of</strong>, Department <strong>of</strong> Biophysical Chemistry, Academy <strong>of</strong> Sciences <strong>of</strong> the Czech Republic,<br />
Prague<br />
D. Russell, Department <strong>of</strong> Microbiology & Immunology, College <strong>of</strong> Veterinary Medicine;<br />
Cornell <strong>University</strong>, Ithaca, USA<br />
I. Parmryd, Cell Biology, The Wenner-Gren <strong>Institute</strong>, Stockholm <strong>University</strong>, Stockholm,<br />
Sweden<br />
T. Hugel, IMETUM, TU München, Garching, Germany<br />
P. Kinnunen, Biophysics, Aalto <strong>University</strong>, Helsinki, Finland<br />
Research projects:<br />
BM.W_F Genomforschung in Österreich (GEN-AU): Exploring the lipolytic proteome.<br />
Phospholipases (GOLD 3 project-Genomics <strong>of</strong> Lipid-associated Disorders, coordinated<br />
by KFU <strong>Graz</strong>).<br />
FWF Special Research Program (SFB) LIPOTOX (coordinated by KFU <strong>Graz</strong>): Toxicity <strong>of</strong><br />
oxidized phospholipids in macrophages.<br />
FWF Doctoral Program Molecular Enzymology: Functional enzyme analysis.<br />
FWF EUROCORES-EuroMEMBRANE-OXPHOS-Protein targets and apoptotic signaling <strong>of</strong><br />
oxidized phospholipids<br />
Invited lectures:<br />
1. Functional proteomic analysis <strong>of</strong> hydrolytic enzymes relevant to the stability and<br />
activity <strong>of</strong> oxidized phospholipids in macrophages. Cellular radical stress and related<br />
biomarkers, COST Chemistry CM0603, Athens, Greece, February 22, 2010<br />
2. Molecular and supramolecular targets <strong>of</strong> oxidized phospholipids. ESF Euromembrane<br />
Meeting, Heidelberg, Germany, April 7, 2010<br />
3. Primary targets <strong>of</strong> oxidized phospholipids in macrophages. 5 th Prague- Wroclaw<br />
Seminar on Biophysics, Prague, Czech Republic, November 18, 2010<br />
Publications:<br />
1. Landre JB, Blaess MF, Kohl M, Schlicksbier T, Ruryk A, Kinscherf R, Claus RA,<br />
Hermetter A, Keller M, Bauer M, Deigner HP<br />
Addressable Bipartite Molecular Hook (ABMH): Immobilized Hairpin Probes with<br />
Sensitivity below 50 Femtomolar<br />
Anal.Biochem. 397, 60-66, 2010.<br />
2. Sattler W, Nusshold C, Kollroser M, Köfeler H, rechberger G, reicher H, Üllen A,<br />
Bernhart E,Waltl S,Kratzer I,Hermetter A,Hackl H, Trajanoski Z,Hrzenjak A, Malle E<br />
Hypochlorite modification <strong>of</strong> sphingomyelin generates chlorinated lipid species that<br />
induce apoptosis and proteome alterations in dopaminergic PC12neurons in vitro<br />
Free Radical Biol Med 48, 1588-1600, 2010.<br />
31
3. Vanderven BC, Hermetter A, Huang A, Maxfield FR, Russell DG, Yates RM<br />
Development <strong>of</strong> a novel, cell-based chemical screen to identify inhibitors <strong>of</strong><br />
intraphagosomal lipolysis in macrophages<br />
Cytometry A 77, 751-760, 2010.<br />
4. Watschinger K, Keller MA, Golderer G, Hermann M, Maglione M, Sarg B, Lindner<br />
HH, Hermetter A, Werner-Felmayer G, konrat R, Hulo, N, Werner ER<br />
Identification <strong>of</strong> the gene encoding alkylglycerol monooxygenase defines a third class <strong>of</strong><br />
tetrahydrobiopterin dependent enzymes<br />
PNAS, 107, 13672-13677, 2010.<br />
5. Plochberger B, Stockner T, Chiantia S, Brameshuber M, Weghuber J, Hermetter A,<br />
Schwille P, Schuetz GJ<br />
Cholesterol slows down the lateral mobility <strong>of</strong> an oxidized phospholipid in a supported<br />
lipid bilayer<br />
Langmuir, 26, 17322-17329, 2010.<br />
6. Schicher M, Morak M, Birner-Gruenberger R, Kayer H, Stojcic B, Rechberger G,<br />
Kollroser M, Hermetter A<br />
Functional proteomic analysis <strong>of</strong> lipases and esterases in cultured human adipocytes<br />
J. Proteome Res., 9, 6334-6344, 2010.<br />
32
Chemistry <strong>of</strong> Functional Foods<br />
Group leader: Michael Murkovic<br />
PhD students: Alam Zeb, Yuliana Reni Swasti<br />
Diploma students: Elham Fanaee Danesch, Tatjana Golubkova, Zivile Rocyte<br />
Technician: Alma Ljubijankic<br />
Honorary Pr<strong>of</strong>essor: Klaus Günther, Forschungszentrum Jülich, Germany<br />
Visiting Pr<strong>of</strong>essors: Evangelos Katzojannos, TEJ <strong>of</strong> Athens, Greece; Zhong Hayan, Central<br />
South <strong>University</strong> <strong>of</strong> Forestry and <strong>Technology</strong>, China<br />
General description<br />
Antioxidants have different functions depending on the location <strong>of</strong> action. Is it the protection<br />
<strong>of</strong> biological systems maintaining the integrity <strong>of</strong> the system or the protection <strong>of</strong> foods against<br />
oxidation leading to health threatening substances The exposure to oxidation products is<br />
either described as oxidative stress or the oxidized substances have an acute or chronic<br />
toxicity or are carcinogenic. The production <strong>of</strong> healthier and safer foods is <strong>of</strong> primary interest<br />
<strong>of</strong> this research group.<br />
The antioxidants <strong>of</strong> interest are polyphenols including anthocyanins and carotenoids. The<br />
evaluation <strong>of</strong> their occurrence in food and their behavior during processing and cooking is<br />
important especially when these substances are used as food additives. The safety evaluation<br />
<strong>of</strong> these compounds includes the evaluation <strong>of</strong> possible degradation products.<br />
Heating <strong>of</strong> food is a process that is normally done to improve the safety and digestibility and<br />
improve the sensory attributes like texture, color, and aroma. During the heating reactions<br />
occur that lead to the degradation <strong>of</strong> nutritive constituents like carbohydrates, proteins, amino<br />
acids and lipids. Some <strong>of</strong> the reaction products are contributing to the nice aroma, color, and<br />
texture <strong>of</strong> the prepared food and many <strong>of</strong> them are highly toxic and/or carcinogenic. However,<br />
these hazardous compounds occur in rather low concentrations being normally not acute<br />
toxic. The substances have a very diverse chemical background like heterocyclic amines,<br />
polycondensated aromatic compounds, acrylamide or furan derivatives. The aim <strong>of</strong> the<br />
research is to investigate the reaction mechanisms that lead to the formation <strong>of</strong> these<br />
hazardous compounds and establish strategies to mitigate the formation and thereby reducing<br />
the alimentary exposure.<br />
Carotenoids thermal oxidation in triacylglycerols<br />
β-Carotene is one <strong>of</strong> most important and widespread carotenoids present in food. It is an<br />
important source <strong>of</strong> provitamin A. It acts as antioxidant in biological systems including the<br />
complex system <strong>of</strong> lipids. We studied the oxidation <strong>of</strong> β-carotene in model triacylglycerol<br />
systems, comparable to most <strong>of</strong> the high oleic edible oil. An HPLC electrospray ionization<br />
mass spectrometric method was developed for the separation and identification <strong>of</strong><br />
triacylglycerols and its oxidation products (Figure 1). β-Carotene was found to act as<br />
antioxidant at lower temperature like 90°C and 100°C, while oxidation <strong>of</strong> triacylglycerols was<br />
closely correlated with the oxidation <strong>of</strong> β-carotene after 8 hours <strong>of</strong> thermal oxidation at 110<br />
°C. The oxidized products <strong>of</strong> β-carotene were epoxides, peroxides and apocarotenals,<br />
separated and analyzed using C30 reversed phase HPLC-DAD and APCI-MS. We found that<br />
110 °C and 120 °C were the favorable temperatures for studying the oxidation reaction <strong>of</strong><br />
carotenoids, while 110 °C, 120 °C and 130 °C were favorable for triacylglycerol oxidation.<br />
33
Trilinolein<br />
263<br />
288<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
599<br />
896<br />
901<br />
1-Oleoyl-2-linoleoyl-3-linoleoyl glycerol<br />
288<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
599<br />
601<br />
O<br />
O<br />
O<br />
O<br />
O<br />
898<br />
+<br />
881<br />
O<br />
872<br />
+<br />
903<br />
Triolein<br />
263<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
1-Oleoyl-2-oleoyl-3-stearoyl glycerol<br />
+<br />
883<br />
+<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
Stability <strong>of</strong> triacylglycerols decreases with the increased formation <strong>of</strong> the oxidation products<br />
<strong>of</strong> β-carotene.<br />
100<br />
1-Oleoyl-2-linoleoyl-3-oleoyl glycerol<br />
[ M + Na]<br />
+<br />
905<br />
603<br />
[ M + ] + NH4<br />
100 OO +<br />
902<br />
80<br />
80<br />
Intensity<br />
60<br />
OL +<br />
601<br />
[ M + NH4]<br />
+<br />
900<br />
Intensity<br />
60<br />
[ M + Na]<br />
+<br />
907<br />
40<br />
20<br />
288<br />
263<br />
0<br />
200 300 400 500 600 700 800 900<br />
m/z<br />
OO +<br />
603<br />
[ M-O +H+ Li]<br />
+<br />
874<br />
+ [ M + H]<br />
883<br />
40<br />
20<br />
[ M + H]<br />
0<br />
200 300 400 500 600 700 800 900<br />
m/z<br />
[ M- O+ H + Li]<br />
876<br />
100<br />
+ [ M + Na]<br />
100<br />
+ [ M + Na]<br />
100<br />
[ M + NH4 ] +<br />
909<br />
[ M + Na]<br />
+<br />
904<br />
80<br />
Intensity<br />
80<br />
60<br />
40<br />
20<br />
[ M + NH 4]<br />
+<br />
+ [ M + H]<br />
879<br />
0<br />
200 300 400 500 600 700 800 900<br />
m/z<br />
LL +<br />
Intensity<br />
80<br />
60<br />
40<br />
20<br />
LL +<br />
[ M + NH4]<br />
+<br />
[ M-O+ Li]<br />
H+<br />
[ M + H]<br />
0<br />
200 300 400 500 600 700 800 900<br />
m/z<br />
OL +<br />
Intensity<br />
60<br />
OO +<br />
603<br />
887<br />
40<br />
20<br />
288 [ M + H<br />
+<br />
]<br />
0<br />
200 300 400 500 600 700 800 900<br />
OS +<br />
605<br />
DAGs<br />
HPLC-MS<br />
0 5 10 15 20 25 30<br />
Time (min)<br />
Figure 1. Separation and Identification <strong>of</strong> Triacylglycerols using HPLC-ESI-MS.<br />
Doctoral Thesis completed<br />
Alam Zeb: Carotenoids and Triacylglycerols Interactions during Oxidation<br />
Carotenoids (β-carotene and astaxanthin) were oxidized in high oleic model triacylglycerols<br />
(TAGs) and edible oils such as corn and olive oils. The main techniques used in this<br />
dissertation were HPTLC and HPLC coupled to DAD and mass spectrometry. The previous<br />
literature on the uses <strong>of</strong> TLC suggests that HPTLC have the potential to be the first choice in<br />
the analysis <strong>of</strong> carotenoids in foods. We also found that HPTLC is a useful tool in the study <strong>of</strong><br />
degradation <strong>of</strong> β-carotene in model TAGs and edible oils. The isocratic HPLC-ESI-MS<br />
method was very useful for the fast screening and identification <strong>of</strong> TAGs in edible oils. We<br />
correctly identify and separated thirteen, fourteen, fifteen and sixteen TAGs in refined olive<br />
oil, rapeseed oil, corn oil and sunflower oil, respectively. The oxidation products <strong>of</strong> TAGs<br />
were also studied using this method. Epoxy epidioxides, hydroxy bis-hydroperoxides and<br />
epidioxy bishydroperoxides were identified as major oxidized compounds that have been<br />
identified for the first time in model TAGs and edible oils under similar conditions. Other<br />
triacylglycerols oxidized species were hydroxy hydroperoxides, mono-hydroperoxides, bishydroperoxides,<br />
epoxy-epidioxides, and epoxides. Significant degradation <strong>of</strong> β-carotene was<br />
observed in sunflower oil. In high oleic model TAGs, β-carotene degraded significantly in the<br />
first three hours, however, in olive oil <strong>of</strong> relatively similar TAG composition, β-carotene<br />
degraded slowly. Astaxanthin degradation was much slower than β-carotene in olive oil. The<br />
HPLC method for the degradation and oxidation <strong>of</strong> carotenoids reveal a total <strong>of</strong> eight oxidized<br />
compounds <strong>of</strong> β-carotene in corn oil. The degradation <strong>of</strong> all-E-β-carotene in corn oil was<br />
relatively similar to model TAGs and olive oil. The interactions <strong>of</strong> carotenoids and TAGs<br />
reveal the pro-oxidant action <strong>of</strong> both β-carotene and astaxanthin. The pro-oxidant action <strong>of</strong> β-<br />
carotene was much stronger than astaxanthin. These findings help us to understand the<br />
34
structural characterization <strong>of</strong> TAGs using mass spectrometry and the possible role and<br />
interactions <strong>of</strong> carotenoids or its oxidation products with the normal and oxidized<br />
triacylglycerols during thermal oxidation.<br />
Master Thesis completed<br />
Elham Fanaee Danesch: Ethanol production from fruit waste with solid state fermentation<br />
using Saccharomyces cerevisiae<br />
Disposal <strong>of</strong> agricultural wastes including rotten fruits and vegetables particularly in large<br />
cities and the lack <strong>of</strong> an appropriate method <strong>of</strong> recycling not only causes environmental<br />
pollution but also needs a loss <strong>of</strong> resources. These wastes are a good source <strong>of</strong> carbohydrates,<br />
acids, fibers, inorganic compounds and vitamins. Therefore they have a good potential for<br />
production <strong>of</strong> ethanol, animal feed, enzymes, pectin, etc. In this research, production <strong>of</strong><br />
ethanol from fruit wastes by Saccharomyces cervisiae was investigated at 28 °C, pH 4.5 and<br />
77 % moisture in solid state fermentation. S. cervisiae was obtained from Biochemical and<br />
Bioenvironmental Research Center (B.B.R.C.). A maximum ethanol production yield <strong>of</strong> 13.75<br />
% based on the initial concentration was obtained from sugar after 22 h <strong>of</strong> fermentation which<br />
is equivalent to 1 g <strong>of</strong> ethanol per 42.35 g <strong>of</strong> fruit waste. The optimum value <strong>of</strong> effective<br />
parameters in production <strong>of</strong> ethanol were found to be 1 % ammonium sulfate, 1.5 %<br />
potassium dihydrogen phosphate, 2 % glucose and 41.06 g fruit waste. At the end <strong>of</strong> the<br />
fermentation process, 71 % <strong>of</strong> the substrate sugar was consumed.<br />
International cooperations<br />
R. Venskutonis, <strong>Institute</strong> <strong>of</strong> Food <strong>Technology</strong>, Kaunas <strong>University</strong> <strong>of</strong> <strong>Technology</strong>, Lithuania<br />
T. Husoy, National <strong>Institute</strong> <strong>of</strong> Public Health, Olso, Norway<br />
H.R. Glatt, Deutsches <strong>Institute</strong> für Ernährungsforschung, Potsdam Rehbrücke, Germany<br />
E. Lazos, TEJ <strong>of</strong> Athens, Greece<br />
R. Grujic, <strong>University</strong> <strong>of</strong> East Sarajevo, Bosnia and Herzegovina<br />
E. Habul, <strong>University</strong> <strong>of</strong> Sarajevo, Bosnia and Herzegovina<br />
E. Winkelhausen, S. Kuzmanova, <strong>University</strong> Ss Cyril and Methodius, Skopie, FRYM<br />
H. Pinheiro, Instituto Superior Tecnico, Lisboa, Portugal<br />
V. Piironen, Department <strong>of</strong> Applied Chemistry and Microbiology, Helsinki, Finland<br />
M.J. Cantalejo, Department <strong>of</strong> Food Technol., Public <strong>University</strong> <strong>of</strong> Navarre, Pamplona, Spain<br />
Z. Cieserova, Food Research <strong>Institute</strong>, Bratislava, Slovakia<br />
C. Thongkraung, Prince <strong>of</strong> Songkla <strong>University</strong>, Hatyai, Thailand<br />
Research project<br />
European Network <strong>of</strong> Excellence: EuroFIR European Food Information Resource<br />
Lectures<br />
1) A. Zeb<br />
ß-Carotene induced oxidation <strong>of</strong> high oleic triacylglycerols model system.<br />
Österreichische Lebensmittelchemikertage. Schloss Seggau-Leibnitz, 19 May 2010<br />
35
2) M. Murkovic<br />
Zusatzst<strong>of</strong>fe - unverzichtbare Begleiter in der Küche<br />
Wissenschaft mit Geschmack. <strong>Graz</strong>, 16 June 2010<br />
3) A. Zeb<br />
Role <strong>of</strong> Astaxanthin in Thermal Oxidation <strong>of</strong> High Oleic Triacylglycerols.<br />
8 th Euro Fed Lipid Congress. Munich, 21 Nov. 2010<br />
Publications<br />
1) Zeb, A.; Murkovic, M.:<br />
Analysis <strong>of</strong> triacylglycerols in refined edible oils by isocratic HPLC-ESI-MS.<br />
European journal <strong>of</strong> lipid science and technology 112 (2010) 8, S. 844 – 851<br />
2) Jöbstl, D.; Husoy, T.; Alexander, J.; Bjellaas, T.; Leitner, E.; Murkovic, M.:<br />
Analysis <strong>of</strong> 5-hydroxymethyl-2-furoic acid (HMFA) the main metabolite <strong>of</strong> alimentary<br />
5-hydroxymethyl-2-furfural (HMF) with HPLC and GC in urine.<br />
Food Chemistry 123 (2010), S. 814 - 818<br />
3) Prasetyo, E. N.; Kudanga, T.; Steiner, W.; Murkovic, M.; Wonisch, W.; Nyanhongo, G.<br />
S.; Gübitz, G.:<br />
Cellular and plasma antioxidant activity assay using tetramethoxy.<br />
Free radical biology & medicine 49 (2010), S. 1205 - 1211<br />
4) Zeb, A.; Murkovic, M.:<br />
Characterization <strong>of</strong> the effects <strong>of</strong> β-Carotene on the thermal oxidation <strong>of</strong><br />
Triacylglycerols using HPLC-ESI-MS.<br />
European journal <strong>of</strong> lipid science and technology 112 (2010) 11, S. 1218 - 1228<br />
5) Zeb, A.; Murkovic, M.:<br />
High-Performance Thin-Layer Chromatographic Method for Monitoring the Thermal<br />
Degradation <strong>of</strong> β-Carotene in Sunflower Oil.<br />
Journal <strong>of</strong> Planar Chromatography - modern TLC 23 (2010) 1, S. 35 - 39<br />
6) Nugroho Prasetyo, E.; Kudanga, T.; Steiner, W.; Murkovic, M.; Nyanhongo, G. S.;<br />
Gübitz, G.:<br />
Laccase-generated tetramethoxy azobismethylene quinone (TMAMQ) as a tool for<br />
antioxidant activity measurement.<br />
Food Chemistry 118 (2010) 2, S. 437 - 444<br />
7) Zeb, A.; Murkovic, M.:<br />
Thin-Layer Chromatographic Analysis <strong>of</strong> Carotenoids in Plant and Animal Samples.<br />
Journal <strong>of</strong> Planar Chromatography - modern TLC 23 (2010) 2, S. 94 - 103<br />
Scientific Conference<br />
Biannual Conference <strong>of</strong> the <strong>Austria</strong>n Food Chemists<br />
<strong>Austria</strong>n Chemical Society – WG Food Chemistry, Cosmetics, and Tensides<br />
19.5. – 21.5.2010, Schloss Seggau, Leibnitz, <strong>Austria</strong><br />
36
Lectures and Laboratory Courses<br />
Aktuelle Trends und Ergebnisse in der<br />
2 VO Günther K<br />
Analytischen Chemie und Lebensmittelchemie<br />
Anleitung zu wissenschaftlichen Arbeiten aus<br />
Biochemie und Molekularer Biomedizin<br />
2 SE Daum G, Hermetter A,<br />
Macheroux P<br />
Anleitung zu wissenschaftlichen Arbeiten aus<br />
Biochemie und Molekularer Biomedizin<br />
2 SE Daum G, Hermetter A,<br />
Macheroux P<br />
Bioanalytik 2.25 VO Hermetter A, Klimant I<br />
Biochemie I 3.75 VO Macheroux P<br />
Biochemie II 1.5 VO Macheroux P<br />
Biophysikalische Chemie 1 2 PV Laggner P<br />
Biophysikalische Chemie 2 2 PV Laggner P<br />
Biophysikalische Chemie der Lipide 1 2 PV Hermetter A<br />
Biophysikalische Chemie der Lipide 2 2 PV Hermetter A<br />
Chemie und Technologie der Lebensmittel II 2 VO Murkovic M<br />
Chemische Veränderungen in Lebensmitteln I 2 PV Murkovic M<br />
Chemische Veränderungen in Lebensmittel II 2 PV Murkovic M<br />
Chemische Veränderungen in Lebensmitteln 2 SE Murkovic M<br />
bei der Verarbeitung<br />
DissertantInnenseminar 1 1 SE Faculty<br />
DissertantInnenseminar 2 1 SE Faculty<br />
Enzymatische und mikrobielle Verfahren in der 2 VO Murkovic M<br />
Lebensmittelherstellung<br />
Fisch- und Fischprodukte 1 VO Murkovic M<br />
Fluoreszenztechnologie 2 VO Hermetter A<br />
Fluoreszenztechnologie 1.5 LU Hermetter A<br />
GL Biochemie (BMT) 2 VO Macheroux P, Waldner-Scott I<br />
GL Chemie (BMT) 2 VO Hermetter A<br />
GL der Pharmakologie 2 VO Dittrich P<br />
Immunologische Methoden 2 VO Daum G<br />
Immunologische Methoden 2 LU Binter A, Daum G, Knaus T,<br />
Wallner S<br />
Immunologische Methoden 1 VO Daum G<br />
Lebensmittelbiotechnologie 1.3 VO Murkovic M<br />
Lebensmittelchemie/Technologie 4 VU Leitner E, Murkovic M<br />
LU aus Biochemie I 5.33 LU Binter A, Daum G, Hermetter<br />
A, Knaus T, Macheroux P,<br />
Waldner-Scott I, Wallner S<br />
LU aus Biochemie II 4 LU Binter A, Daum G, Macheroux<br />
P, Waldner-Scott I, Wallner S<br />
LU aus Molekularbiologie 3 LU Knaus T, Waldner-Scott I<br />
Membran Biophysik 1 2 PV Lohner K<br />
Membran Biophysik 2 2 PV Lohner K<br />
Membran-Mimetik 1 VO Lohner K<br />
Molekulare Enzymologie 2 VO Gruber K, Macheroux P,<br />
Nidetzky B<br />
Molekulare Enzymologie I 2 PV Macheroux P<br />
Molekulare Enzymologie II 2 PV Macheroux P<br />
Molekulare Gastronomie und 1 EV Murkovic M<br />
37
Lebensmittelverarbeitung II<br />
Molekulare Physiologie 2 VO Macheroux P<br />
Physikalische Methoden in der Biochemie 2 LU Laggner P<br />
Physikalische Methoden in der Biochemie 1 VO Laggner P<br />
Projektlabor Bioanalytik 1 6 PR Daum G, Hermetter A,<br />
Macheroux P<br />
Projektlabor Bioanalytik 2 6 PR Daum G, Hermetter A,<br />
Macheroux P<br />
Projektlabor Biochemie und Molekulare<br />
Biomedizin 1<br />
Projektlabor Biochemie und Molekulare<br />
Biomedizin 2<br />
9 PR Trajanoski Z, Macheroux P,<br />
Daum G, Hermetter A,<br />
Waldner-Scott I<br />
9 PR Daum G, Hermetter A,<br />
Macheroux P, Trajanoski Z,<br />
Waldner-Scott I<br />
Projektlabor Lebensmittelbiotechnologie 1 6 PR Leitner E, Murkovic M<br />
Projektlabor Lebensmittelbiotechnologie 2 6 PR Leitner E, Murkovic M<br />
Seminar zu den LU aus Molekularbiologie 1 SE Athenstaedt K, Waldner-Scott I<br />
Spezielle Kapitel der Biochemie 1 VO Daum G, Hermetter A,<br />
Macheroux P<br />
Spezielle Kapitel der Lebensmittelchem.- u. 2 SE Murkovic M<br />
technologie I<br />
Spezielle Kapitel der Lebensmittelchemie- und 2 SE Murkovic M<br />
techn.II<br />
Strukturanalyse in Biophysik u.<br />
2 VO Laggner P<br />
Materialforschung<br />
Unterrichtspraxis 2 SE Faculty<br />
Unterrichtspraxis 2 SE Faculty<br />
Wissenschaftliches Kolloquium für<br />
1 SE Faculty<br />
DissertantInnen 1<br />
Wissenschaftliches Kolloquium für<br />
1 SE Faculty<br />
DissertantInnen 2<br />
Zellbiologie 1.5 VO Daum G<br />
Zellbiologie 1 1 SE Daum G<br />
Zellbiologie 2 1 SE Daum G<br />
Zellbiologie der Lipide 1 2 PV Daum G<br />
Zellbiologie der Lipide 2 2 PV Daum G<br />
38