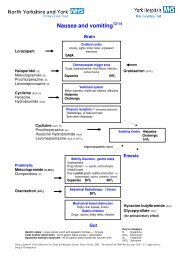
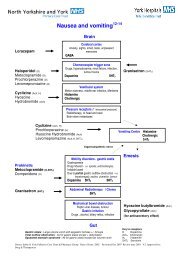
Prescribing information KETAMINE use in chronic pain
Prescribing information KETAMINE use in chronic pain
Prescribing information KETAMINE use in chronic pain
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Prescrib<strong>in</strong>g</strong> <strong><strong>in</strong>formation</strong><br />
<strong>KETAMINE</strong> <strong>use</strong> <strong>in</strong> <strong>chronic</strong> pa<strong>in</strong><br />
This <strong><strong>in</strong>formation</strong> is <strong>in</strong>tended to support hospital doctors prescrib<strong>in</strong>g ketam<strong>in</strong>e for <strong>chronic</strong> pa<strong>in</strong> under<br />
the supervision of the Palliative Care Team. Ketam<strong>in</strong>e is unlicensed for <strong>use</strong> <strong>in</strong> pa<strong>in</strong> and the patient’s GP<br />
would not normally be asked to take on prescrib<strong>in</strong>g. This document may however also serve as a<br />
<strong>use</strong>ful GP reference if one of their patients is ma<strong>in</strong>ta<strong>in</strong>ed on ketam<strong>in</strong>e by the Palliative Care Team<br />
Cl<strong>in</strong>ical Use<br />
Ketam<strong>in</strong>e is a short act<strong>in</strong>g anaesthetic agent that has analgesic properties at subanaesthetic<br />
doses. A synergistic effect between ketam<strong>in</strong>e and opioids has been observed<br />
<strong>in</strong> patients who have lost an analgesic response to high doses of morph<strong>in</strong>e.<br />
Ketam<strong>in</strong>e is usually <strong>use</strong>d <strong>in</strong> pa<strong>in</strong> that has failed to respond fully to opioids despite escalat<strong>in</strong>g<br />
doses and comb<strong>in</strong>ation with appropriate adjuvants. It may be particularly helpful <strong>in</strong><br />
neuropathic pa<strong>in</strong>.<br />
Mode of action<br />
Various mechanisms have been proposed to expla<strong>in</strong> the analgesic effect of ketam<strong>in</strong>e. The<br />
effect seems to be mediated <strong>in</strong> part through <strong>in</strong>hibition of N-methyl-D-aspartate receptors.<br />
Ketam<strong>in</strong>e also <strong>in</strong>teracts with chol<strong>in</strong>ergic and opiate receptors and possibly <strong>in</strong>hibits the<br />
synaptic re-uptake of monoam<strong>in</strong>es.<br />
Formulation<br />
Ketam<strong>in</strong>e is usually adm<strong>in</strong>istered orally, or occasionally by subcutaneous <strong>in</strong>fusion <strong>in</strong> a<br />
syr<strong>in</strong>ge driver.<br />
Start<strong>in</strong>g oral ketam<strong>in</strong>e<br />
1. If the patient is already tak<strong>in</strong>g an opioid a dose reduction should be considered e.g. up<br />
to 30 to 50% dose reduction. Occasionally it may be necessary to change patients<br />
from controlled release morph<strong>in</strong>e to an immediate release formulation.<br />
2. A typical start<strong>in</strong>g dose of oral ketam<strong>in</strong>e is 10 to 25mg every 6-8 hours and prn<br />
3. Consider the <strong>use</strong> of oral haloperidol 1.5mg to 3mg at night or oral diazepam 5mg for<br />
neuropsychiatic side effects.<br />
4. Doses are usually <strong>in</strong>creased <strong>in</strong> steps of 10 to 25 mg per dose every 3-4 days until the<br />
desired dose is reached up to a usual maximum of 50mg every 6 hours<br />
(maximum reported dose 200mg every 6 hours).<br />
5. Titrate the opioid dose down further if possible<br />
6. If pa<strong>in</strong> is return<strong>in</strong>g before the next dose is due the dos<strong>in</strong>g <strong>in</strong>terval can be shortened to<br />
every 4-6 hours.<br />
Notes on availability<br />
• The only licensed commercially available formulation of ketam<strong>in</strong>e is an <strong>in</strong>jection<br />
(50mg/mL and 100mg/mL multidose vials). The <strong>in</strong>jection may be given orally us<strong>in</strong>g a<br />
syr<strong>in</strong>ge to measure the dose, it does however have a bitter taste that may be disguised<br />
with flavour<strong>in</strong>gs such as orange juice and cola dr<strong>in</strong>ks. The 50mg/mL is less bitter than<br />
100mg/mL and should be <strong>use</strong>d <strong>in</strong> preference. For convenience the pharmacist supply<strong>in</strong>g<br />
the ketam<strong>in</strong>e may be prepared to draw the <strong>in</strong>jection out of the vials and supply it <strong>in</strong> a<br />
bottle with an oral syr<strong>in</strong>ge. This however is an unlicensed transaction<br />
• An unlicensed oral solution is available from Mart<strong>in</strong>dale <strong>in</strong> various strengths.<br />
Ketam<strong>in</strong>e <strong>in</strong> pa<strong>in</strong> (professional) 1
Start<strong>in</strong>g ketam<strong>in</strong>e s/c <strong>in</strong>fusion<br />
1. If the patient is already tak<strong>in</strong>g an opioid a dose reduction should be considered e.g. up<br />
to 30 to 50% dose reduction. Occasionally it may be necessary to change patients<br />
from a controlled release morph<strong>in</strong>e to an immediate release formulation.<br />
2. Give a load<strong>in</strong>g dose of 10mg ketam<strong>in</strong>e subcutaneously (diluted <strong>in</strong> 1mL sodium chloride)<br />
3. Commence a subcutaneous syr<strong>in</strong>ge driver conta<strong>in</strong><strong>in</strong>g 50-150mg ketam<strong>in</strong>e over 24<br />
hours.<br />
4. Consider the <strong>use</strong> of haloperidol 1.5mg to 3mg (s/c <strong>in</strong> syr<strong>in</strong>ge driver over 24hrs or orally)<br />
or midazolam 5-10mg (s/c <strong>in</strong> syr<strong>in</strong>ge driver over 24hrs) for neuropsychiatic side effects.<br />
5. Doses are usually <strong>in</strong>creased by 50mg every 24 hours until the desired dose is reached<br />
up to a usual maximum dose of 700mg over 24 hours.<br />
6. Titrate the opioid dose down further if possible<br />
Note<br />
• Subcutaneous ketam<strong>in</strong>e can be irritant and if <strong>use</strong>d alone is probably best diluted with<br />
sodium chloride 0.9%. If mix<strong>in</strong>g with other drugs <strong>use</strong> water for <strong>in</strong>jection for dilution.<br />
• Use a dilute solution i.e <strong>use</strong> of a 20mL or 30mL syr<strong>in</strong>ge<br />
• Ketam<strong>in</strong>e is compatible with diamorph<strong>in</strong>e, metoclopramide, haloperidol, midazolam,<br />
metoclopramide, levomepromaz<strong>in</strong>e<br />
• Ketam<strong>in</strong>e may be <strong>in</strong>compatible with cycliz<strong>in</strong>e and some doses of dexamethasone<br />
Chang<strong>in</strong>g patients from subcutaneous to oral ketam<strong>in</strong>e<br />
1. The dose of ketam<strong>in</strong>e orally is approximately half of that given subcutaneously<br />
2. Prescribe the appropriate oral dose <strong>in</strong> 3 divided doses.<br />
3. For the first 24 hours cont<strong>in</strong>ue the subcutaneous route but at 50% of the orig<strong>in</strong>al dose<br />
4. Increase the oral dose by 10-25mg daily every 3-4 days<br />
5. If pa<strong>in</strong> is return<strong>in</strong>g before the next dose is due the dos<strong>in</strong>g <strong>in</strong>terval can be shortened to<br />
every 4-6 hours.<br />
Notes on availability of oral ketam<strong>in</strong>e<br />
See previous page (start<strong>in</strong>g oral ketam<strong>in</strong>e)<br />
Monitor<strong>in</strong>g of patients<br />
Consider monitor<strong>in</strong>g respiration rate, blood pressure, heart rate, sedation and pa<strong>in</strong> every 4<br />
hours for the first 24 hours and for 24 hours follow<strong>in</strong>g any dose change.<br />
Pharmacok<strong>in</strong>etics<br />
The onset of action of ketam<strong>in</strong>e is 15-30 m<strong>in</strong>utes after subcutaneous adm<strong>in</strong>istration and 30<br />
m<strong>in</strong>utes after oral adm<strong>in</strong>istration.<br />
Ketam<strong>in</strong>e is poorly absorbed after oral adm<strong>in</strong>istration and is also subject to first pass<br />
metabolism. Oral bioavailability is therefore only 20%. Ketam<strong>in</strong>e is metabolised <strong>in</strong> the liver<br />
by the cytochrome P45 enzyme system. CYP3A4 has been shown to be the major enzyme<br />
responsible and CYP2B6 and CYP2C9 play a m<strong>in</strong>or role. Chronic ketam<strong>in</strong>e adm<strong>in</strong>istration<br />
<strong>in</strong>creases the activity of the enzymes <strong>in</strong>volved <strong>in</strong> its own metabolism, and this may modify<br />
the response with repeated adm<strong>in</strong>istration and may be responsible for drug <strong>in</strong>teractions with<br />
concurrently adm<strong>in</strong>istered drugs that are metabolised by the same enzymes. The ma<strong>in</strong><br />
metabolite norketam<strong>in</strong>e does however also have analgesic properties and is produced <strong>in</strong><br />
greater quantities than ketam<strong>in</strong>e result<strong>in</strong>g <strong>in</strong> a oral:subcutaneous dos<strong>in</strong>g ratio of between<br />
2:1 and 4:1. Tolerance has been reported.<br />
Ketam<strong>in</strong>e <strong>in</strong> pa<strong>in</strong> (professional) 2
Less than 10% of the drug is excreted unchanged , 5% <strong>in</strong> the faeces and 5% by the<br />
kidneys. The biologically active metabolite, norketam<strong>in</strong>e, is subject to hydroxylation and<br />
conjugation before be<strong>in</strong>g excreted by the kidneys. Impaired renal function does not prolong<br />
the effect of the drug.<br />
Drug <strong>in</strong>teractions<br />
Ketam<strong>in</strong>e may potentially <strong>in</strong>teract with other drugs metabolised by cytochrome P450.<br />
CYP3A4 has been shown to be the major enzyme responsible and CYP2B6 and CYP2C9<br />
play a m<strong>in</strong>or role. The plasma concentration of ketam<strong>in</strong>e may be <strong>in</strong>creased by diazepam.<br />
Side effects<br />
Neuropsychiatric side effects such as dysphoria, halluc<strong>in</strong>ations and nightmares may occur<br />
early <strong>in</strong> therapy, but tolerance usually occurs rapidly. If necessary these can be reduced<br />
by concurrent treatment with haloperidol or a benzodiazep<strong>in</strong>e. Care must however be<br />
taken as benzodiazep<strong>in</strong>es can <strong>in</strong>crease the amount of available ketam<strong>in</strong>e and may also<br />
enhance the respiratory depressant effects.<br />
Other side effects <strong>in</strong>cludes sedation, confusion, <strong>in</strong>creased muscle tone, disorientation,<br />
delirium and dizz<strong>in</strong>ess, and if encountered require patients reassurance.<br />
Side effects associated with higher doses and may warrant dose reduction <strong>in</strong>clude<br />
tachycardia, hypertension, diplopia and nystagmus.<br />
Contra-<strong>in</strong>dications<br />
Absolute : Intracranial hypertension, seizures, neurological impairment<br />
Relative : Hypertension, cardiac failure, previous CVA<br />
Patient <strong><strong>in</strong>formation</strong><br />
Patients should be issued with a Trust patient <strong><strong>in</strong>formation</strong> leaflet (Ketam<strong>in</strong>e <strong>in</strong> pa<strong>in</strong>)<br />
<strong>Prescrib<strong>in</strong>g</strong> responsibility<br />
As the <strong>use</strong> of ketam<strong>in</strong>e <strong>in</strong> pa<strong>in</strong> is unlicensed, treatment will normally cont<strong>in</strong>ue to be supplied<br />
by the Consultant <strong>in</strong>itiat<strong>in</strong>g treatment.<br />
Cost (Mims Aug 2007)<br />
Ketam<strong>in</strong>e <strong>in</strong>jection 50mg/mL (10mL multidose vial) £ 8.77<br />
Ketam<strong>in</strong>e <strong>in</strong>jection 100mg/mL (10mL multidose vial) £16.10<br />
Ketam<strong>in</strong>e solution ( unlicensed from Mart<strong>in</strong>dale) 50mg/5mL 500mL<br />
£112 (<strong>in</strong>c VAT)<br />
References<br />
1. Mercadante S. Ketam<strong>in</strong>e <strong>in</strong> cancer pa<strong>in</strong>, an update. Palliative Medic<strong>in</strong>e 1996;10:225-230<br />
2. Broadley KE et al. Ketam<strong>in</strong>e <strong>in</strong>jection <strong>use</strong>d orally. Palliative Medic<strong>in</strong>e 1996;10:247-250<br />
3. Luczak J et al. The role of ketam<strong>in</strong>e an NMDA receptor antagonist <strong>in</strong> the management of pa<strong>in</strong>. Progress <strong>in</strong> Palliative<br />
Care 1995;3:127-134<br />
4. Dickman A et al..The Syr<strong>in</strong>ge Driver. Oxford University press 2002<br />
5. The Palliative care Formulary PCF 2<br />
6. Bell RF et al. Ketam<strong>in</strong>e as adjuvant to opioids for cancer pa<strong>in</strong>. A qualitative systematic review. Journal of Pa<strong>in</strong> and<br />
Symptom Management 2003; 26(3): 867-875.<br />
Prepared by : Palliative care pharmacy team - available from Jane Crewe (Pharmacy)<br />
Approved by : York Interface D&T Committee 10/05<br />
Date of preparation : 10/05 Reviewed: 08/07<br />
Date of review : Version 3 Review date: 8/2010<br />
Ketam<strong>in</strong>e <strong>in</strong> pa<strong>in</strong> (professional) 3