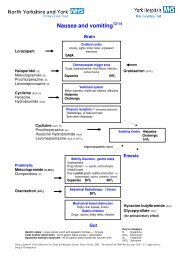
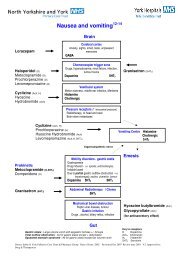
Anticipatory medicines in palliative care pro-forma V1, review date ...
Anticipatory medicines in palliative care pro-forma V1, review date ...
Anticipatory medicines in palliative care pro-forma V1, review date ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
CONTROLLED DRUG PRESCRIPTION REQUIREMENTS<br />
The follow<strong>in</strong>g MUST be HANDWRITTEN by the prescriber on a CD prescription:<br />
Patient’s name, address and hospital number<br />
Drug name (generic and branded if ap<strong>pro</strong>priate)<br />
Strength of preparation (e.g. 10mg/5mL)<br />
Form (e.g. liquid, capsules, tablets, <strong>in</strong>jection)<br />
Quantity to supply <strong>in</strong> words and figures e.g. 56 (fifty six) tablets<br />
Dose and Dosage <strong>in</strong>terval (e.g. 20mg 4 to 6 hourly)<br />
Prescriber’s signature, bleep, pr<strong>in</strong>ted name and <strong>date</strong><br />
Version number: 1 Date active: 29 th March 2012 Review <strong>date</strong>: 29 th March 2014<br />
Author: Amanda Crockett Check by: Chemotherapy Development Group + Ap<strong>pro</strong>ved by: Drug and Therapeutics Committee Page 2 of 2<br />
Palliative Care Team