Oxidation of benzoin catalyzed by oxovanadium(IV) schiff base ...
Oxidation of benzoin catalyzed by oxovanadium(IV) schiff base ...
Oxidation of benzoin catalyzed by oxovanadium(IV) schiff base ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Alsalim et al. Chemistry Central Journal 2013, 7:3 Page 6 <strong>of</strong> 8<br />
http://journal.chemistrycentral.com/content/7/1/3<br />
Yield (%)<br />
100<br />
80<br />
60<br />
40<br />
20<br />
MeCN<br />
CCl4<br />
Benzene<br />
0<br />
0 2 4 6 8 10<br />
Time (h)<br />
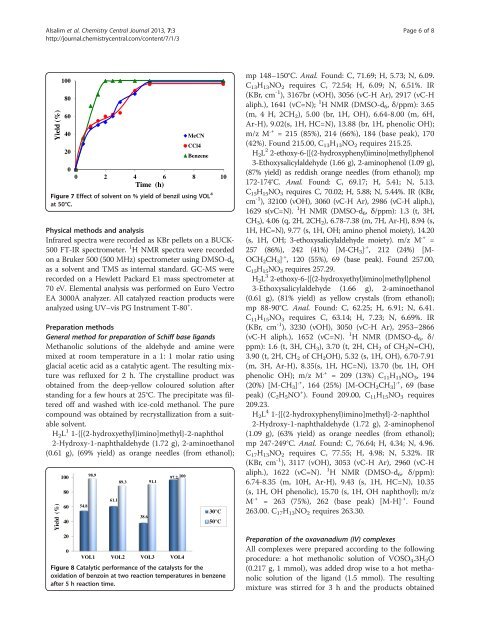
Figure 7 Effect <strong>of</strong> solvent on % yield <strong>of</strong> benzil using VOL 4<br />
at 50°C.<br />
Physical methods and analysis<br />
Infrared spectra were recorded as KBr pellets on a BUCK-<br />
500 FT-IR spectrometer. 1 H NMR spectra were recorded<br />
on a Bruker 500 (500 MHz) spectrometer using DMSO-d 6<br />
as a solvent and TMS as internal standard. GC-MS were<br />
recorded on a Hewlett Packard E1 mass spectrometer at<br />
70 eV. Elemental analysis was performed on Euro Vectro<br />
EA 3000A analyzer. All <strong>catalyzed</strong> reaction products were<br />
analyzed using UV–vis PG Instrument T-80 + .<br />
Preparation methods<br />
General method for preparation <strong>of</strong> Schiff <strong>base</strong> ligands<br />
Methanolic solutions <strong>of</strong> the aldehyde and amine were<br />
mixed at room temperature in a 1: 1 molar ratio using<br />
glacial acetic acid as a catalytic agent. The resulting mixture<br />
was refluxed for 2 h. The crystalline product was<br />
obtained from the deep-yellow coloured solution after<br />
standing for a few hours at 25°C. The precipitate was filtered<br />
<strong>of</strong>f and washed with ice-cold methanol. The pure<br />
compound was obtained <strong>by</strong> recrystallization from a suitable<br />
solvent.<br />
H 2 L 1 1-{[(2-hydroxyethyl)imino]methyl}-2-naphthol<br />
2-Hydroxy-1-naphthaldehyde (1.72 g), 2-aminoethanol<br />
(0.61 g), (69% yield) as orange needles (from ethanol);<br />
Yield (%)<br />
100<br />
80<br />
60<br />
40<br />
20<br />
0<br />
54.8<br />
98.9<br />
61.1<br />
89.3 91.1<br />
38.6<br />
97.2 100<br />
VOL1 VOL2 VOL3 VOL4<br />
30°C<br />
50°C<br />
Figure 8 Catalytic performance <strong>of</strong> the catalysts for the<br />
oxidation <strong>of</strong> <strong>benzoin</strong> at two reaction temperatures in benzene<br />
after 5 h reaction time.<br />
mp 148–150°C. Anal. Found: C, 71.69; H, 5.73; N, 6.09.<br />
C 13 H 13 NO 2 requires C, 72.54; H, 6.09; N, 6.51%. IR<br />
(KBr, cm -1 ), 3167br (νOH), 3056 (νC-H Ar), 2917 (νC-H<br />
aliph.), 1641 (νC=N); 1 H NMR (DMSO-d 6 , δ/ppm): 3.65<br />
(m, 4 H, 2CH 2 ), 5.00 (br, 1H, OH), 6.64-8.00 (m, 6H,<br />
Ar-H), 9.02(s, 1H, HC=N), 13.88 (br, 1H, phenolic OH);<br />
m/z M .+ = 215 (85%), 214 (66%), 184 (<strong>base</strong> peak), 170<br />
(42%). Found 215.00, C 13 H 13 NO 2 requires 215.25.<br />
H 2 L 2 2-ethoxy-6-{[(2-hydroxyphenyl)imino]methyl}phenol<br />
3-Ethoxysalicylaldehyde (1.66 g), 2-aminophenol (1.09 g),<br />
(87% yield) as reddish orange needles (from ethanol); mp<br />
172-174°C. Anal. Found: C, 69.17; H, 5.41; N, 5.13.<br />
C 15 H 15 NO 3 requires C, 70.02; H, 5.88; N, 5.44%. IR (KBr,<br />
cm -1 ), 32100 (νOH), 3060 (νC-H Ar), 2986 (νC-H aliph.),<br />
1629 s(νC=N).<br />
1 H NMR (DMSO-d 6 , δ/ppm): 1.3 (t, 3H,<br />
CH 3 ), 4.06 (q, 2H, 2CH 2 ), 6.78-7.38 (m, 7H, Ar-H), 8.94 (s,<br />
1H,HC=N),9.77(s,1H,OH;aminophenolmoiety),14.20<br />
(s, 1H, OH; 3-ethoxysalicylaldehyde moiety). m/z M .+ =<br />
257 (86%), 242 (41%) [M-CH 3 ] .+ , 212 (24%) [M-<br />
OCH 2 CH 3 ] .+ , 120 (55%), 69 (<strong>base</strong> peak). Found 257.00,<br />
C 15 H 15 NO 3 requires 257.29.<br />
H 2 L 3 2-ethoxy-6-{[(2-hydroxyethyl)imino]methyl}phenol<br />
3-Ethoxysalicylaldehyde (1.66 g), 2-aminoethanol<br />
(0.61 g), (81% yield) as yellow crystals (from ethanol);<br />
mp 88-90°C. Anal. Found: C, 62.25; H, 6.91; N, 6.41.<br />
C 11 H 15 NO 3 requires C, 63.14; H, 7.23; N, 6.69%. IR<br />
(KBr, cm -1 ), 3230 (νOH), 3050 (νC-H Ar), 2953–2866<br />
(νC-H aliph.), 1652 (νC=N).<br />
1 H NMR (DMSO-d 6 , δ/<br />
ppm): 1.6 (t, 3H, CH 3 ), 3.70 (t, 2H, CH 2 <strong>of</strong> CH 2 N=CH),<br />
3.90 (t, 2H, CH 2 <strong>of</strong> CH 2 OH), 5.32 (s, 1H, OH), 6.70-7.91<br />
(m, 3H, Ar-H), 8.35(s, 1H, HC=N), 13.70 (br, 1H, OH<br />
phenolic OH); m/z M .+ = 209 (13%) C 11 H 15 NO 3 , 194<br />
(20%) [M-CH 3 ] .+ , 164 (25%) [M-OCH 2 CH 3 ] .+ , 69 (<strong>base</strong><br />
peak) (C 2 H 5 NO + ). Found 209.00, C 11 H 15 NO 3 requires<br />
209.23.<br />
H 2 L 4 1-{[(2-hydroxyphenyl)imino]methyl}-2-naphthol<br />
2-Hydroxy-1-naphthaldehyde (1.72 g), 2-aminophenol<br />
(1.09 g), (63% yield) as orange needles (from ethanol);<br />
mp 247-249°C. Anal. Found: C, 76.64; H, 4.34; N, 4.96.<br />
C 17 H 13 NO 2 requires C, 77.55; H, 4.98; N, 5.32%. IR<br />
(KBr, cm -1 ), 3117 (νOH), 3053 (νC-H Ar), 2960 (νC-H<br />
aliph.), 1622 (νC=N).<br />
1 H NMR (DMSO-d 6 , δ/ppm):<br />
6.74-8.35 (m, 10H, Ar-H), 9.43 (s, 1H, HC=N), 10.35<br />
(s, 1H, OH phenolic), 15.70 (s, 1H, OH naphthoyl); m/z<br />
M .+ = 263 (75%), 262 (<strong>base</strong> peak) [M-H] .+ . Found<br />
263.00. C 17 H 13 NO 2 requires 263.30.<br />
Preparation <strong>of</strong> the oxavanadium (<strong>IV</strong>) complexes<br />
All complexes were prepared according to the following<br />
procedure: a hot methanolic solution <strong>of</strong> VOSO 4 .3H 2 O<br />
(0.217 g, 1 mmol), was added drop wise to a hot methanolic<br />
solution <strong>of</strong> the ligand (1.5 mmol). The resulting<br />
mixture was stirred for 3 h and the products obtained