Soil Generic Assessment Criteria for Human Health Risk ... - ESdat
Soil Generic Assessment Criteria for Human Health Risk ... - ESdat
Soil Generic Assessment Criteria for Human Health Risk ... - ESdat
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
www.esdat.net Esdat Environmental Database Management Software +61 2 8875 7948<br />
1,1,2 trichloroethane<br />
TDI oral<br />
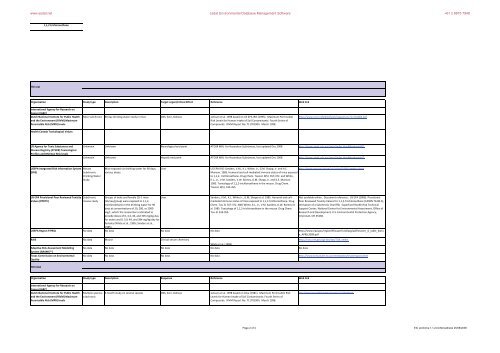
Organisation<br />
Study type Description Target organ/Critical Effect Reference Web link<br />
International Agency <strong>for</strong> Research on<br />
Cancer (IARC)<br />
Dutch National Institute <strong>for</strong> Public <strong>Health</strong><br />
and the Environment (RIVM) Maximum<br />
Permissible <strong>Risk</strong> (MPR) levels.<br />
<strong>Health</strong> Canada Toxicological Values<br />
Mice subchronic 90 day drinking water study in mice CNS, liver, kidneys Janssen et al. 1998 based on US EPA IRIS (1995). Maximum Permissible<br />
<strong>Risk</strong> Levels <strong>for</strong> <strong>Human</strong> Intake of <strong>Soil</strong> Contaminants: Fourth Series of<br />
Compounds. RIVM Report No. 711701004. March 1998.<br />
http://www.rivm.nl/bibliotheek/rapporten/711701004.pdf<br />
US Agency <strong>for</strong> Toxic Substances and<br />
Disease Registry (ATSDR) Toxicological<br />
Profiles and Minimal <strong>Risk</strong> levels<br />
Unknown Unknown Neurological end point ATDSR MRL <strong>for</strong> Hazardous Substances, last updated Dec 2008 http://www.atsdr.cdc.gov/mrls/index.html#bookmark02<br />
Unknown Unknown Hepatic end point ATDSR MRL <strong>for</strong> Hazardous Substances, last updated Dec 2008 http://www.atsdr.cdc.gov/mrls/index.html#bookmark02<br />
USEPA Integrated <strong>Risk</strong> In<strong>for</strong>mation System<br />
(IRIS)<br />
Mouse<br />
Subchronic<br />
Drinking Water<br />
Study<br />
Mice exposed via drinking water <strong>for</strong> 90 days,<br />
various doses.<br />
Liver<br />
US EPA IRIS: Sanders, V.M., K.L. White, Jr., G.M. Shopp, Jr. and A.E. http://www.epa.gov/ncea/iris/subst/0198.htm#studoral<br />
Munson. 1985. Humoral and cell-mediated immune status of mice exposed<br />
to 1,1,2- trichloroethane. Drug Chem. Toxicol. 8(5): 357-372. and White,<br />
K.L., Jr., V.M. Sanders, V.W. Barnes, G.M. Shopp, Jr. and A.E. Munson.<br />
1985. Toxicology of 1,1,2-trichloroethane in the mouse. Drug Chem.<br />
Toxicol. 8(5): 333-355.<br />
US EPA Provisional Peer Reviewed Toxicity<br />
Values (PPRTV)<br />
USEPA Region 9 PRGs<br />
RAIS<br />
Adaptive <strong>Risk</strong> <strong>Assessment</strong> Modelling<br />
System (ARAMS)<br />
Texas Commission on Environmental<br />
Quality<br />
TDI inhal<br />
Subchronic<br />
mouse study<br />
Groups of male and female CD-1 mice<br />
(16/sex/group) were exposed to 1,1,2-<br />
trichloroethane in the drinking water <strong>for</strong> 90<br />
days at concentrations of 20, 200, or 2000<br />
mg/L, which the researchers estimated to<br />
provide doses of 0, 4.4, 46, and 305 mg/kg-day<br />
Liver<br />
Sanders, V.M., K.L. White Jr., G.M. Shopp et al. 1985. Humoral and cellmediated<br />
immune status of mice exposed to 1,1,2-trichloroethane. Drug<br />
Chem. Tox. 8: 357-372. AND White, K.L. Jr., V.M. Sanders, D.W. Barnes et<br />
al. 1985. Toxicology of 1,1,2-trichloroethane in the mouse. Drug Chem.<br />
Tox. 8: 333-355.<br />
Not available online. Document reference: US EPA (2006). Provisional<br />
Peer Reviewed Toxicity Values <strong>for</strong> 1,1,2-Trichloroethane (CASRN 79-00-5),<br />
Derivation of a Subchronic Oral RfD, Superfund <strong>Health</strong> <strong>Risk</strong> Technical<br />
Support Center, National Center <strong>for</strong> Environmental <strong>Assessment</strong>, Office of<br />
Research and Development, U.S. Environmental Protection Agency,<br />
Cincinnati, OH 45268.<br />
<strong>for</strong> males and 0, 3.9, 44, and 384 mg/kg-day <strong>for</strong><br />
females (White et al., 1985; Sanders et al.,<br />
1985).<br />
No data No data No data No data http://www.epa.gov/region09/superfund/prg/pdf/master_sl_table_bwru<br />
n_APRIL2009.pdf<br />
No data Mouse Clinical serum chemistry<br />
http://rais.ornl.gov/cgi-bin/tox/TOX_select<br />
White et al. (1985)<br />
No data No data No data No data No data<br />
No data No data No data No data http://www.tceq.state.tx.us/remediation/trrp/trrppcls.html<br />
Organisation<br />
Study type Description Response Reference Web link<br />
International Agency <strong>for</strong> Research on<br />
Cancer (IARC)<br />
Dutch National Institute <strong>for</strong> Public <strong>Health</strong><br />
and the Environment (RIVM) Maximum<br />
Permissible <strong>Risk</strong> (MPR) levels<br />
Multiple species,<br />
subchronic<br />
6 month study on several species CNS, liver, kidneys Janssen et al. 1998 based on Dow (1981). Maximum Permissible <strong>Risk</strong><br />
Levels <strong>for</strong> <strong>Human</strong> Intake of <strong>Soil</strong> Contaminants: Fourth Series of<br />
Compounds. RIVM Report No. 711701004. March 1998.<br />
http://www.rivm.nl/bibliotheek/rapporten/711701004.pdf<br />
Page 2 of 4 EIC pro<strong>for</strong>ma 1,1,2-trichloroethane 25/08/2009