Soil Generic Assessment Criteria for Human Health Risk ... - ESdat
Soil Generic Assessment Criteria for Human Health Risk ... - ESdat
Soil Generic Assessment Criteria for Human Health Risk ... - ESdat
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
www.esdat.net Esdat Environmental Database Management Software +61 2 8875 7948<br />
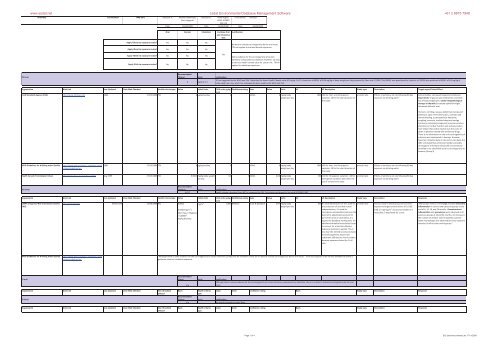
Antimony CASNumber: 7440360 AssessorA: AndreasNeymeyer,<br />
BuroHappold<br />
AssessorB:<br />
MikeRogers,<br />
OPUSJOYNES<br />
PIKELtd<br />
Finalreview:<br />
Panel/SF<br />
Date 16/04/2009 Date 16/04/2009 Date 26/08/2009<br />
Oral Dermal Inhalation CombineOral<br />
andInhalation<br />
TDIs<br />
Justification<br />
ApplyTDIoraltoexposureroutes<br />
ApplyIDoraltoexposureroutes<br />
Yes Yes No<br />
No No No<br />
Nodatatoindicatecarcinogenicitybytheoralroute.<br />
TDIoralappledtooralanddermalexposures.<br />
ApplyTDIinhtoexposureroutes<br />
ApplyIDinhtoexposureroutes<br />
No No Yes<br />
No No No<br />
Yes<br />
Someevidence<strong>for</strong>thecarcinogenicityofcertain<br />
antimonycompoundsbyinhalation;however,nodata<br />
toderiveahealthcriteriavalue<strong>for</strong>cancerrisk.TDIinh<br />
appliedtoinhalationexposures.<br />
TDIoral<br />
Recommended<br />
TDIoral<br />
Units<br />
Justification<br />
6 ug.kg1.d1<br />
TDIassuggestedbytheWHOandFSA.Notethatthelower<strong>Health</strong>Canadavalue(0.2ug.kg1.d1)isbasedonaNOAELof0.06mg/kgofbodyweightperdayproposedbyPoonetal.(1998).ThisNOAELwasquestionedbyLynchetal.(1999)whopreferredaNOAELof6.0mg/kgof<br />
bodyweightperday,whichhassubsequentlybeenusedbytheWHOandFSA.<br />
ggg<br />
Organisation WebLink LastUpdated DateWebChecked <strong>Health</strong>criteriatype Value InitialUnits CLEAunitsug.kg Confidencerating Basis Value Units UF UFdescription Studytype Description Targetorgan/CriticalEffect<br />
1.d1<br />
FoodStandardsAgency(FSA) http://www.food.gov.uk/ 2003 27/03/2009 TDI 6 g/kgbw/day 6 NOAEL 6 mg/kgbody<br />
weightperday<br />
Effectsofantimonyonratsfollowing90day<br />
exposureviadrinkingwater<br />
1000 100<strong>for</strong>interandintraspecies animal(rats)<br />
variation,10<strong>for</strong>theshortdurationof<br />
thestudy<br />
Animalstudies:decreasedlongevityandaltered<br />
bloodlevelsofglucoseandcholesterol,reversible<br />
lossofbodyweightgain,subtlehistopathological<br />
changesinthyroids(increasedepithelialheight,<br />
decreasedfollicularsize)<br />
<strong>Human</strong>s:vomiting,nausea,abdominalcrampsand<br />
diarrhoea,opticnervedestruction,uveitidesand<br />
retinalbleeding,accompaniedbyheadache,<br />
coughing,anorexia,troubledsleepandvertigo<br />
Antimonycontainingcompoundsmayalsoproduce<br />
alterationsincardiacfunctionandautopsystudies<br />
haveshownthatcardiactoxicitywasthecauseof<br />
deathinpatientstreatedwithantimonialdrugs.<br />
Thereisnoin<strong>for</strong>mationontheoralcarcinogenicityof<br />
antimonyandcompoundsinhumans.However<br />
basedoninhalationdatainratsandinvitrodata,the<br />
IARCconcludedthatantimonytrioxideispossibly<br />
carcinogenictohumans(Group2B),andantimony<br />
trisulfideisnotclassifiableastoitscarcinogenicityto<br />
humans(Group3)<br />
WHOGuidelines<strong>for</strong>drinkingwaterQuality<br />
http://www.who.int/water_sanitation_healt<br />
h/dwq/guidelines/en<br />
2003 27/03/2009 TDI 6 g/kgbw/day 6 NOAEL 6 mg/kgbody<br />
weightperday<br />
1000 100<strong>for</strong>interandintraspecies animal(rats)<br />
variation,10<strong>for</strong>theshortdurationof<br />
thestudy<br />
Effectsofantimonyonratsfollowing90day<br />
exposureviadrinkingwater<br />
<strong>Health</strong>CanadaToxicologicalValues http://www.hcsc.gc.ca/index_e.html Aug1999 27/03/2009 TDI 0.0002 mg/kgbodyweight<br />
perday<br />
0.2 3 NOAEL 0.06 mg/kgbody<br />
weightperday<br />
300 ×10<strong>for</strong>intraspeciesvariation;×10<strong>for</strong><br />
interspeciesvariation;and×3<strong>for</strong>the<br />
useofashorttermstudy<br />
animal(rats)<br />
Effectsofantimonyonratsfollowing90day<br />
exposureviadrinkingwater<br />
TDIinhal<br />
Recommended<br />
TDIinhal<br />
Units<br />
Justification<br />
0.057<br />
ggg<br />
<strong>Health</strong>criteriacalculatedfromRfCassuggestedbyIRIS.Calculationper<strong>for</strong>medinlinewithSC050021/SR2<br />
Organisation WebLink LastUpdated DateWebChecked <strong>Health</strong>criteriatype Value InitialUnits CLEAunitsug.kg Confidencerating Basis Value Units UF UFdescription Studytype Description Response<br />
1.d1<br />
USEPAIntegrated<strong>Risk</strong>In<strong>for</strong>mationSystem<br />
(IRIS)<br />
http://epa.gov/iris/ 09/01/1995 12/08/2009 RfC 0.0002<br />
mg/m 3 0.057 Medium Study&Database 0.07 mg/kgbody<br />
weightperday<br />
(0.0002mg/m 3 x<br />
20m 3 /day/70kg(bw)<br />
=0.00007<br />
mg/kgbw/day)<br />
300 Anuncertaintyfactorof10isused<strong>for</strong> animal(rats)<br />
theprotectionofsensitivehuman<br />
subpopulations,3isused<strong>for</strong><br />
interspeciesextrapolationbecausethe<br />
dosimetricadjustmentsaccount<strong>for</strong><br />
partofthisareaofuncertainty,3is<br />
applied<strong>for</strong>databaseinadequacies.An<br />
additionalthreefolduncertaintyfactor<br />
toaccount<strong>for</strong>alessthanlifetime<br />
exposuredurationisapplied.Thisis<br />
lessthanthe10folduncertaintyfactor<br />
normallyappliedtoadjustfrom<br />
subchronic(90day)tochronicstudies<br />
becauseexposureslasted<strong>for</strong>1full<br />
year.<br />
Chronicstudyinwhichgroupsofratswere<br />
exposedtotargetconcentrationsof0,0.05,<br />
0.50,or5.00mg/m 3 antimonytrioxide<strong>for</strong>6<br />
hours/day,5days/week<strong>for</strong>1year.<br />
"Microscopiclesionsofthelungsrevealedinterstitial<br />
inflammationincontrolandexposuregroupsatthe<br />
endof6,12,18,and24months. Granulomatous<br />
inflammationandgranulomaswereobservedinall<br />
exposuregroupsat18and24months.Anincreasein<br />
thenumberofalveolarandintraalveolarparticle<br />
ladenmacrophageswasobserved(ateveryexposure<br />
duration)inallbutthecontrolgroups."<br />
WHOGuidelines<strong>for</strong>drinkingwaterQuality<br />
http://www.who.int/water_sanitation_healt<br />
h/dwq/guidelines/en<br />
"Althoughthereissomeevidence<strong>for</strong>thecarcinogenicityofcertainantimonycompoundsbyinhalation,therearenodatatoindicatecarcinogenicitybytheoralroute."Therealsoappearstobenodataavailabletoderivea<br />
guidancevalue<strong>for</strong>inhalationexposure.<br />
IDoral<br />
Organisation WebLink LastUpdated DateWebChecked Nonthreshold<br />
effects<br />
IDinhal<br />
Organisation WebLink LastUpdated DateWebChecked Nonthreshold<br />
effects<br />
Recommended<br />
IDoral Units Justification<br />
Althoughthereissomeevidence<strong>for</strong>thecarcinogenicityofcertainantimonycompoundsbyinhalation,thereisnodatatoindicatecarcinogenicitybytheoral<br />
n/a<br />
ggg<br />
route.<br />
Basis<br />
<strong>Health</strong>criteria Value Units Confidencerating<br />
Basis<br />
Studytype Description Response<br />
type<br />
Recommended<br />
IDinhal<br />
Units<br />
Justification<br />
n/a<br />
Nodata<strong>for</strong>inhalationIndexDose<br />
ggg<br />
Basis<br />
<strong>Health</strong>criteria Value Units Confidencerating<br />
Basis<br />
Studytype Description Response<br />
type<br />
Page 1 of 4<br />
EIC pro<strong>for</strong>ma antimony.xls 17/11/2009