Soil Generic Assessment Criteria for Human Health Risk ... - ESdat
Soil Generic Assessment Criteria for Human Health Risk ... - ESdat Soil Generic Assessment Criteria for Human Health Risk ... - ESdat
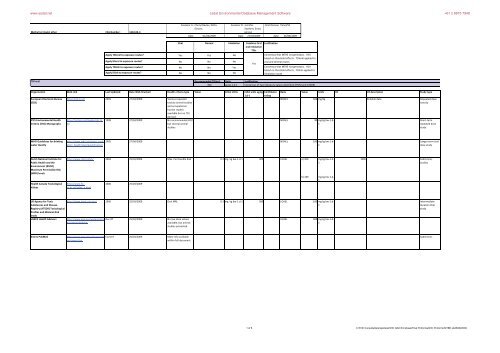
www.esdat.net Esdat Environmental Database Management Software +61 2 8875 7948 Methyl tert-butyl ether CAS Number: 1634-04-4 Apply TDIoral to exposure routes Apply IDoral to exposure routes Apply TDIinh to exposure routes Apply IDinh to exposure routes Assessor A: Cheryl Davies, Delta- Simons Assessor B: Jennifer Stothert, Entec UK Ltd Final Review: Panel/SF Date 01/03/2009 Date 27/04/2009 date: 26/08/2009 Oral Dermal Inhalation Combine Oral and Inhalation TDIs Justification Yes Yes No Consensus that MTBE not genotoxic. HCV based on threshold effects. TDIoral applied to No No No Yes oral and dermal routes No No Yes Consensus that MTBE not genotoxic. HCV based on threshold effects. TDIinh applied to No No No inhalation route TDI oral Recommended TDIoral Units 300 ug.kg-1.d-1 Justification Consensus of two literature values identified (RIVM and ATSDR) Organisation Web Link Last Updated Date Web Checked Health criteria type Value Initial Units CLEA units ug.kg- Confidence 1.d-1 rating European Chemicals Bureau http://ecb.jrc.it/ 2002 27/04/2009 Various repeated (ECB) toxicity animal studies and occupational human studies available but no TDI derived. IPCS Environmental Health Criteria (EHC) Monographs http://inchem.org/pages/ehc.ht ml 1998 27/04/2009 No recommended HCV but several animal studies Basis Value Units UF UF description Study type NOAEL 300 mg/kg Reliable data Repeated dose toxicity NOAEL 90 mg.kg bw-1.d- 1 Short-term repeated dose study WHO Guidelines for drinking water Quality http://www.who.int/water_sanit ation_health/dwq/guidelines/en 2005 27/04/2009 NOAEL 100 mg.kg bw-1.d- 1 Longer term oral dose study Dutch National Institute for Public Health and the Environment (RIVM) Maximum Permissible Risk (MPR) levels Health Canada Toxicological Values http://www.rivm.nl/en/ 2004 25/03/2009 Max. Permissible Risk 0.3 mg. kg bw-1.d-1 300 LOAEL a) 300 http://www.hcsc.gc.ca/index_e.html 2006 25/03/2009 b) 200 mg.kg bw-1.d- 1 mg.kg bw-1.d- 1 1000 Subchronic studies US Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles and Minimal Risk levels USEPA Health Advisors http://www.atsdr.cdc.gov/ 1996 25/03/2009 Oral MRL 0.3 mg. kg bw-1.d-1 300 LOAEL 100 mg.kg bw-1.d- 1 http://www.epa.gov/waterscienc e/criteria/drinking Dec-97 25/03/2009 No low dose values available, but animal studies presented LOAEL 300 mg.kg bw-1.d- 1 Intermediate duration Oral study Entrez PubMed http://www.ncbi.nlm.nih.gov/ent rez/query.fcgi Current 26/03/2009 More info available within full document Subchronic 1 of 6 C:\Firth Consultants\projects\eic\EIC GAC\3rd phase\Final Proformas\EIC Proforma MTBE.xls26/08/2009
www.esdat.net Esdat Environmental Database Management Software +61 2 8875 7948 Methyl tert-butyl ether TDI oral Organisation Description Target organ/Critical Effect Reference Web link European Chemicals Bureau (ECB) Oral NOAEL of 300 mg/kg based on a) 90 day study of Sprague-Dawley rats b) Male rat study a) Elevated AST with weight increase at 900 mg/kg b) Slight morphological liver abnormalities at 200 mg/kg a) Robinson M, Bruner RH & Olson GR (1990). Fourteen- and ninety-day oral toxicity studies of methyl tertiary-butyl ether in Sprague-Dawley rats. Journal of the American College of Toxicology, 9, 525-540. b) Zhou W & Ye S (1999). Subchronic Oral Methyl Ter http://ecb.jrc.ec.europa.eu/Documents/Existing- Chemicals/RISK_ASSESSMENT/REPORT/mtbereport313.pdf IPCS Environmental Health Criteria (EHC) Monographs WHO Guidelines for drinking water Quality Dutch National Institute for Public Health and the Environment (RIVM) Maximum Permissible Risk (MPR) levels In a 28-day oral study, Sprague-Dawley rats (10/sex/group) were administered 0, 90, 440 or 1750 mg undiluted MTBE (purity not specified)/kg bw daily by gavage for a total of 20 h (IITRI, 1992). In a 90 day study groups of 10 male and female rats were administered 0, 100,300, 900 or 1200 mg.kg bw-1. d-1 of MTBE in corn oil a) 90 day oral by gavage study in rats b) 90 day oral by gavage study in rats Increased kidney weights IITRI (1992) 28-day oral (gavage) toxicity of methyl tert-butyl ether (MTBE) in rats (Project No. L08100). Chicago, Illinois, Illinois Institute of Technology Research, 48 pp. Increase in relative kidney weight a) Kidney and liver toxicity b) Increased liver and kidney weight Robinson M, Bruner RH, Olson GR (1990) Fourteen- and ninety-day oral toxicity studies of methyl tertiary-butyl ether in Sprague-Dawley rats. Journal of the American College of Toxicology, 9: 525– 540. TDI within ECB (2002) based on a) and b). ECB (2002). European Union Risk Assessment Report t-butyl methyl ether. European Chemicals Bureau, 3rd Priority list, volume 19; Office for Official Publications of the EC, Luxembourg. a) Robinson M, Bruner RH, Ol http://inchem.org/documents/ehc/ehc/ehc206.htm#SubSectionNumber:7 .4.1 http://www.who.int/water_sanitation_health/dwq/chemicals/MTBE2006 05.pdf http://www.rivm.nl/bibliotheek/rapporten/711701039.pdf Health Canada Toxicological Values Health Canada has determined that there exist too many uncertainties and limitations in the MTBE database to have confidence in a quantitative risk assessment for human health. Guidelines for Canadian Drinking Water Quality: Guideline Technical Document. Methyl Tertiary-Butyl Ether (MTBE). Health Canada, Ottawa, Ontario, July 2006 http://www.hc-sc.gc.ca/ewh-semt/alt_formats/hecs-sesc/pdf/pubs/watereau/mtbe/mtbe-eng.pdf US Agency for Toxic Substances and Disease Registry (ATSDR) Toxicological Profiles and Minimal Risk levels USEPA Health Advisors Entrez PubMed 90 day study Hepatic effects Robinson M, Bruner RH, Olson GR (1990) Fourteen- and ninety-day oral toxicity studies of methyl tertiary-butyl ether in Sprague-Dawley rats. Journal of the American College of Toxicology, 9: 525– 540. 90 day oral exposure study in rats Kidney effects Robinson M, Bruner RH, Olson GR (1990) Fourteen- and ninety-day oral toxicity studies of methyl tertiary-butyl ether in Sprague-Dawley rats. Journal of the American College of Toxicology, 9: 525– 540. MTBE administered to rats at doses of 0,400,800 and 1600 mg. Kg bw-1.d-1. Relative change in heart, liver, kidney, testes, thymus and prostate weight Effects of subchronic methyl tert-butyl ether ether exposure on male Sprague-Dawley rats. Dong-Mei L, Yi G, Chun-Tao Y, Yu-Feng H, Xiao-Dong H. Toxicol Ind Health. 2009 Feb;25(1):15-23. http://www.atsdr.cdc.gov/toxprofiles/tp91.pdf http://www.epa.gov/waterscience/criteria/drinking/mtbe.pdf http://www.ncbi.nlm.nih.gov/pubmed/19318501ordinalpos=2&itool=Ent rezSystem2.PEntrez.Pubmed.Pubmed_ResultsPanel.Pubmed_DefaultRepo rtPanel.Pubmed_RVDocSum 2 of 6 C:\Firth Consultants\projects\eic\EIC GAC\3rd phase\Final Proformas\EIC Proforma MTBE.xls26/08/2009
- Page 227 and 228: www.esdat.net Esdat Environmental D
- Page 229 and 230: www.esdat.net Esdat Environmental D
- Page 231 and 232: www.esdat.net Esdat Environmental D
- Page 233 and 234: www.esdat.net Esdat Environmental D
- Page 235 and 236: www.esdat.net Esdat Environmental D
- Page 237 and 238: www.esdat.net Esdat Environmental D
- Page 239 and 240: www.esdat.net Esdat Environmental D
- Page 241 and 242: www.esdat.net Esdat Environmental D
- Page 243 and 244: www.esdat.net Esdat Environmental D
- Page 245 and 246: www.esdat.net Esdat Environmental D
- Page 247 and 248: www.esdat.net Esdat Environmental D
- Page 249 and 250: www.esdat.net Esdat Environmental D
- Page 251 and 252: www.esdat.net Esdat Environmental D
- Page 253 and 254: www.esdat.net Esdat Environmental D
- Page 255 and 256: www.esdat.net Esdat Environmental D
- Page 257 and 258: www.esdat.net Esdat Environmental D
- Page 259 and 260: www.esdat.net Esdat Environmental D
- Page 261 and 262: www.esdat.net Esdat Environmental D
- Page 263 and 264: www.esdat.net Esdat Environmental D
- Page 265 and 266: www.esdat.net Esdat Environmental D
- Page 267 and 268: www.esdat.net Esdat Environmental D
- Page 269 and 270: www.esdat.net Esdat Environmental D
- Page 271 and 272: www.esdat.net Esdat Environmental D
- Page 273 and 274: www.esdat.net Esdat Environmental D
- Page 275 and 276: www.esdat.net Esdat Environmental D
- Page 277: www.esdat.net Esdat Environmental D
- Page 281 and 282: www.esdat.net Esdat Environmental D
- Page 283 and 284: www.esdat.net Esdat Environmental D
- Page 285 and 286: www.esdat.net Esdat Environmental D
- Page 287 and 288: www.esdat.net Esdat Environmental D
- Page 289 and 290: www.esdat.net Esdat Environmental D
- Page 291 and 292: www.esdat.net Esdat Environmental D
- Page 293 and 294: www.esdat.net Esdat Environmental D
- Page 295 and 296: www.esdat.net Esdat Environmental D
- Page 297 and 298: www.esdat.net Esdat Environmental D
- Page 299 and 300: www.esdat.net Esdat Environmental D
- Page 301 and 302: www.esdat.net Esdat Environmental D
- Page 303 and 304: www.esdat.net Esdat Environmental D
- Page 305 and 306: www.esdat.net Esdat Environmental D
- Page 307 and 308: www.esdat.net Esdat Environmental D
- Page 309 and 310: www.esdat.net Esdat Environmental D
- Page 311 and 312: www.esdat.net Esdat Environmental D
- Page 313 and 314: www.esdat.net Esdat Environmental D
- Page 315 and 316: www.esdat.net Esdat Environmental D
- Page 317 and 318: www.esdat.net Esdat Environmental D
- Page 319 and 320: www.esdat.net Esdat Environmental D
- Page 321: www.esdat.net Esdat Environmental D
www.esdat.net Esdat Environmental Database Management Software +61 2 8875 7948<br />
Methyl tert-butyl ether CAS Number: 1634-04-4<br />
Apply TDIoral to exposure routes<br />
Apply IDoral to exposure routes<br />
Apply TDIinh to exposure routes<br />
Apply IDinh to exposure routes<br />
Assessor A: Cheryl Davies, Delta-<br />
Simons<br />
Assessor B: Jennifer<br />
Stothert, Entec<br />
UK Ltd<br />
Final Review: Panel/SF<br />
Date 01/03/2009 Date 27/04/2009 date: 26/08/2009<br />
Oral Dermal Inhalation Combine Oral<br />
and Inhalation<br />
TDIs<br />
Justification<br />
Yes Yes No<br />
Consensus that MTBE not genotoxic. HCV<br />
based on threshold effects. TDIoral applied to<br />
No No No<br />
Yes<br />
oral and dermal routes<br />
No No Yes<br />
Consensus that MTBE not genotoxic. HCV<br />
based on threshold effects. TDIinh applied to<br />
No No No<br />
inhalation route<br />
TDI oral Recommended TDIoral Units<br />
300 ug.kg-1.d-1<br />
Justification<br />
Consensus of two literature values identified (RIVM and ATSDR)<br />
Organisation Web Link Last Updated Date Web Checked <strong>Health</strong> criteria type Value Initial Units CLEA units ug.kg- Confidence<br />
1.d-1<br />
rating<br />
European Chemicals Bureau http://ecb.jrc.it/ 2002 27/04/2009 Various repeated<br />
(ECB)<br />
toxicity animal studies<br />
and occupational<br />
human studies<br />
available but no TDI<br />
derived.<br />
IPCS Environmental <strong>Health</strong><br />
<strong>Criteria</strong> (EHC) Monographs<br />
http://inchem.org/pages/ehc.ht<br />
ml<br />
1998 27/04/2009 No recommended HCV<br />
but several animal<br />
studies<br />
Basis Value Units UF UF description Study type<br />
NOAEL 300 mg/kg Reliable data Repeated dose<br />
toxicity<br />
NOAEL<br />
90 mg.kg bw-1.d-<br />
1<br />
Short-term<br />
repeated dose<br />
study<br />
WHO Guidelines <strong>for</strong> drinking<br />
water Quality<br />
http://www.who.int/water_sanit<br />
ation_health/dwq/guidelines/en<br />
2005 27/04/2009 NOAEL 100 mg.kg bw-1.d-<br />
1<br />
Longer term oral<br />
dose study<br />
Dutch National Institute <strong>for</strong><br />
Public <strong>Health</strong> and the<br />
Environment (RIVM)<br />
Maximum Permissible <strong>Risk</strong><br />
(MPR) levels<br />
<strong>Health</strong> Canada Toxicological<br />
Values<br />
http://www.rivm.nl/en/ 2004 25/03/2009 Max. Permissible <strong>Risk</strong> 0.3 mg. kg bw-1.d-1 300 LOAEL a) 300<br />
http://www.hcsc.gc.ca/index_e.html<br />
2006 25/03/2009<br />
b) 200<br />
mg.kg bw-1.d-<br />
1<br />
mg.kg bw-1.d-<br />
1<br />
1000 Subchronic<br />
studies<br />
US Agency <strong>for</strong> Toxic<br />
Substances and Disease<br />
Registry (ATSDR) Toxicological<br />
Profiles and Minimal <strong>Risk</strong><br />
levels<br />
USEPA <strong>Health</strong> Advisors<br />
http://www.atsdr.cdc.gov/ 1996 25/03/2009 Oral MRL 0.3 mg. kg bw-1.d-1 300 LOAEL 100 mg.kg bw-1.d-<br />
1<br />
http://www.epa.gov/waterscienc<br />
e/criteria/drinking<br />
Dec-97 25/03/2009 No low dose values<br />
available, but animal<br />
studies presented<br />
LOAEL<br />
300 mg.kg bw-1.d-<br />
1<br />
Intermediate<br />
duration Oral<br />
study<br />
Entrez PubMed<br />
http://www.ncbi.nlm.nih.gov/ent<br />
rez/query.fcgi<br />
Current 26/03/2009 More info available<br />
within full document<br />
Subchronic<br />
1 of 6 C:\Firth Consultants\projects\eic\EIC GAC\3rd phase\Final Pro<strong>for</strong>mas\EIC Pro<strong>for</strong>ma MTBE.xls26/08/2009