Modeling 17α-ethinylestradiol removal in membrane bioreactors
Modeling 17α-ethinylestradiol removal in membrane bioreactors
Modeling 17α-ethinylestradiol removal in membrane bioreactors
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Poster Session<br />
WWTmod2012<br />
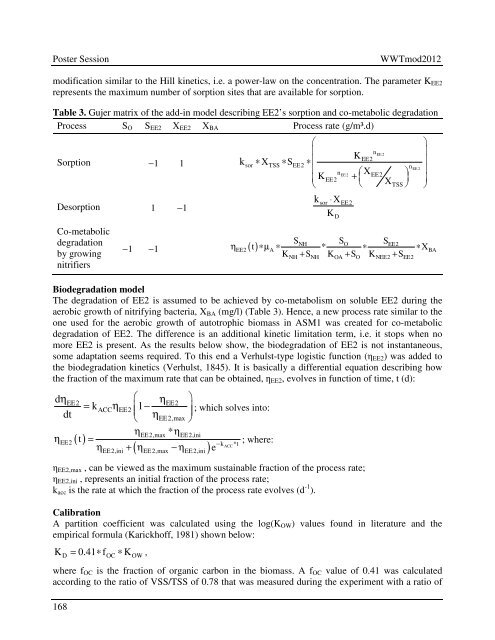
modification similar to the Hill k<strong>in</strong>etics, i.e. a power-law on the concentration. The parameter K EE2<br />
represents the maximum number of sorption sites that are available for sorption.<br />
Table 3. Gujer matrix of the add-<strong>in</strong> model describ<strong>in</strong>g EE2’s sorption and co-metabolic degradation<br />
Process S O S EE2 X EE2 X BA Process rate (g/m³.d)<br />
Sorption − 1 1<br />
Desorption 1 − 1<br />
Co-metabolic<br />
degradation<br />
by grow<strong>in</strong>g<br />
nitrifiers<br />
168<br />
− 1 1<br />
k<br />
⎛<br />
⎜<br />
EE 2<br />
EE2<br />
∗ X ∗S<br />
∗⎜<br />
⎟<br />
⎜<br />
EE 2<br />
n X<br />
EE 2 ⎛ ⎞ ⎟<br />
sor TSS EE2 n<br />
EE2<br />
⎜ KEE2<br />
+ ⎜ X ⎟<br />
TSS<br />
⎝<br />
k<br />
sor<br />
⋅ X<br />
K<br />
− ( )<br />
NH O EE2<br />
D<br />
EE2<br />
S S S<br />
η t ∗µ ∗ * ∗ ∗X<br />
EE2 A BA<br />
KNH + SNH KOA + SO KNEE2 + SEE2<br />
Biodegradation model<br />
The degradation of EE2 is assumed to be achieved by co-metabolism on soluble EE2 dur<strong>in</strong>g the<br />
aerobic growth of nitrify<strong>in</strong>g bacteria, X BA (mg/l) (Table 3). Hence, a new process rate similar to the<br />
one used for the aerobic growth of autotrophic biomass <strong>in</strong> ASM1 was created for co-metabolic<br />
degradation of EE2. The difference is an additional k<strong>in</strong>etic limitation term, i.e. it stops when no<br />
more EE2 is present. As the results below show, the biodegradation of EE2 is not <strong>in</strong>stantaneous,<br />
some adaptation seems required. To this end a Verhulst-type logistic function (η EE2 ) was added to<br />
the biodegradation k<strong>in</strong>etics (Verhulst, 1845). It is basically a differential equation describ<strong>in</strong>g how<br />
the fraction of the maximum rate that can be obta<strong>in</strong>ed, ƞ EE2 , evolves <strong>in</strong> function of time, t (d):<br />
dη<br />
⎛<br />
EE2<br />
η ⎞<br />
EE2<br />
= kACCηEE2<br />
1−<br />
dt<br />
⎜<br />
⎟ ; which solves <strong>in</strong>to:<br />
⎝ ηEE2,max<br />
⎠<br />
ηEE2,max<br />
* ηEE2,<strong>in</strong>i<br />
η t = ; where:<br />
η + η − η<br />
( )<br />
ACC<br />
( ) e −<br />
EE2 k *t<br />
EE2,<strong>in</strong>i EE2,max EE2,<strong>in</strong>i<br />
η EE2,max , can be viewed as the maximum susta<strong>in</strong>able fraction of the process rate;<br />
η EE2,<strong>in</strong>i , represents an <strong>in</strong>itial fraction of the process rate;<br />
k acc is the rate at which the fraction of the process rate evolves (d -1 ).<br />
Calibration<br />
A partition coefficient was calculated us<strong>in</strong>g the log(K OW ) values found <strong>in</strong> literature and the<br />
empirical formula (Karickhoff, 1981) shown below:<br />
KD = 0.41∗fOC ∗ KOW<br />
,<br />
where f OC is the fraction of organic carbon <strong>in</strong> the biomass. A f OC value of 0.41 was calculated<br />
accord<strong>in</strong>g to the ratio of VSS/TSS of 0.78 that was measured dur<strong>in</strong>g the experiment with a ratio of<br />
K<br />
⎝<br />
n<br />
⎠<br />
⎞<br />
⎟<br />
⎟<br />
⎠