English - Focus Diagnostics
English - Focus Diagnostics
English - Focus Diagnostics
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
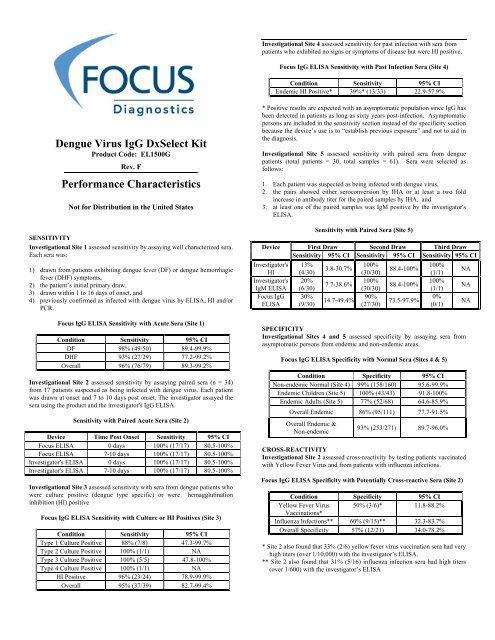
Investigational Site 4 assessed sensitivity for past infection with sera from<br />
patients who exhibited no signs or symptoms of disease but were HI positive.<br />
<strong>Focus</strong> IgG ELISA Sensitivity with Past Infection Sera (Site 4)<br />
Condition Sensitivity 95% CI<br />
Endemic HI Positive* 39%* (13/33) 22.9-57.9%<br />
Dengue Virus IgG DxSelect Kit<br />
Product Code: EL1500G<br />
Rev. F<br />
Performance Characteristics<br />
Not for Distribution in the United States<br />
SENSITIVITY<br />
Investigational Site 1 assessed sensitivity by assaying well characterized sera.<br />
Each sera was:<br />
1) drawn from patients exhibiting dengue fever (DF) or dengue hemorrhagic<br />
fever (DHF) symptoms,<br />
2) the patient’s initial primary draw,<br />
3) drawn within 1 to 16 days of onset, and<br />
4) previously confirmed as infected with dengue virus by ELISA, HI and/or<br />
PCR.<br />
<strong>Focus</strong> IgG ELISA Sensitivity with Acute Sera (Site 1)<br />
Condition Sensitivity 95% CI<br />
DF 98% (49/50) 89.4-99.9%<br />
DHF 93% (27/29) 77.2-99.2%<br />
Overall 96% (76/79) 89.3-99.2%<br />
Investigational Site 2 assessed sensitivity by assaying paired sera (n = 34)<br />
from 17 patients suspected as being infected with dengue virus. Each patient<br />
was drawn at onset and 7 to 10 days post onset. The investigator assayed the<br />
sera using the product and the investigator's IgG ELISA.<br />
Sensitivity with Paired Acute Sera (Site 2)<br />
Device Time Post Onset Sensitivity 95% CI<br />
<strong>Focus</strong> ELISA 0 days 100% (17/17) 80.5-100%<br />
<strong>Focus</strong> ELISA 7-10 days 100% (17/17) 80.5-100%<br />
Investigator's ELISA 0 days 100% (17/17) 80.5-100%<br />
Investigator's ELISA 7-10 days 100% (17/17) 80.5-100%<br />
Investigational Site 3 assessed sensitivity with sera from dengue patients who<br />
were culture positive (dengue type specific) or were hemagglutination<br />
inhibition (HI) positive<br />
<strong>Focus</strong> IgG ELISA Sensitivity with Culture or HI Positives (Site 3)<br />
Condition Sensitivity 95% CI<br />
Type 1 Culture Positive 88% (7/8) 47.3-99.7%<br />
Type 2 Culture Positive 100% (1/1) NA<br />
Type 3 Culture Positive 100% (5/5) 47.8-100%<br />
Type 4 Culture Positive 100% (1/1) NA<br />
HI Positive 96% (23/24) 78.9-99.9%<br />
Overall 95% (37/39) 82.7-99.4%<br />
* Positive results are expected with an asymptomatic population since IgG has<br />
been detected in patients as long as sixty years post-infection. Asymptomatic<br />
persons are included in the sensitivity section instead of the specificity section<br />
because the device’s use is to “establish previous exposure” and not to aid in<br />
the diagnosis.<br />
Investigational Site 5 assessed sensitivity with paired sera from dengue<br />
patients (total patients = 30, total samples = 61). Sera were selected as<br />
follows:<br />
1. Each patient was suspected as being infected with dengue virus,<br />
2. the pairs showed either seroconversion by IHA or at least a two fold<br />
increase in antibody titer for the paired samples by IHA, and<br />
3. at least one of the paired samples was IgM positive by the investigator's<br />
ELISA.<br />
Sensitivity with Paired Sera (Site 5)<br />
Device First Draw Second Draw Third Draw<br />
Sensitivity 95% CI Sensitivity 95% CI Sensitivity 95% CI<br />
Investigator's 13%<br />
100%<br />
100%<br />
3.8-30.7%<br />
88.4-100%<br />
HI (4/30)<br />
(30/30)<br />
(1/1)<br />
NA<br />
Investigator's 20%<br />
100%<br />
100%<br />
7.7-38.6%<br />
88.4-100%<br />
IgM ELISA (6/30)<br />
(30/30)<br />
(1/1)<br />
NA<br />
<strong>Focus</strong> IgG<br />
ELISA<br />
30%<br />
(9/30)<br />
14.7-49.4%<br />
90%<br />
(27/30)<br />
73.5-97.9%<br />
0%<br />
(0/1)<br />
SPECIFICITY<br />
Investigational Sites 4 and 5 assessed specificity by assaying sera from<br />
asymptomatic persons from endemic and non-endemic areas.<br />
<strong>Focus</strong> IgG ELISA Specificity with Normal Sera (Sites 4 & 5)<br />
Condition Specificity 95% CI<br />
Non-endemic Normal (Site 4) 99% (158/160) 95.6-99.9%<br />
Endemic Children (Site 5) 100% (43/43) 91.8-100%<br />
Endemic Adults (Site 5) 77% (52/68) 64.6-85.9%<br />
Overall Endemic 86% (95/111) 77.7-91.5%<br />
Overall Endemic &<br />
Non-endemic<br />
93% (253/271) 89.7-96.0%<br />
CROSS-REACTIVITY<br />
Investigational Site 2 assessed cross-reactivity by testing patients vaccinated<br />
with Yellow Fever Virus and from patients with influenza infections.<br />
<strong>Focus</strong> IgG ELISA Specificity with Potentially Cross-reactive Sera (Site 2)<br />
Condition Specificity 95% CI<br />
Yellow Fever Virus 50% (3/6)* 11.8-88.2%<br />
Vaccinations*<br />
Influenza Infections** 60% (9/15)** 32.3-83.7%<br />
Overall Specificity 57% (12/21) 34.0-78.2%<br />
* Site 2 also found that 33% (2/6) yellow fever virus vaccination sera had very<br />
high titers (over 1/10,000) with the investigator’s ELISA.<br />
** Site 2 also found that 31% (5/16) influenza infection sera had high titers<br />
(over 1/600) with the investigator’s ELISA<br />
NA
FOCUS <strong>Diagnostics</strong><br />
Investigational Site 8 assessed cross-reactivity by testing sera from patients<br />
infected with other flaviviruses, and sera from patients infected with other<br />
viruses. Both serum panels were assayed using the <strong>Focus</strong> device, the<br />
investigator's HI, and the investigator's IgM ELISA. The flavivirus serum<br />
panel consisted of 33 sera from patients infected with other flaviviruses:<br />
Yellow Fever virus, West Nile virus, Chinkunyungunya virus, Japanese<br />
encephalitis virus, Sandfly fever virus, Langat virus, Bunya virus, and<br />
Wassalbron virus.<br />
Cross-reactivity with Flavivirus Infections (Site 8)<br />
Device Cross-reactivity 95% CI<br />
Investigator's Dengue 39% (13/33) 22.9-57.9%<br />
Type 2 HI<br />
Investigator's Dengue 12% (4/32) 3.5-29.0%<br />
ELISA IgM<br />
<strong>Focus</strong> Dengue IgG<br />
ELISA<br />
61% (20/33) 42.1-77.1%<br />
<strong>Focus</strong> IgG ELISA Cross-reactivity<br />
with Non-flavivirus Viral Infections (Site 8)<br />
Device<br />
Cross-reactivity<br />
EBV VCA CMV HSV VZV Parvovirus Overall<br />
Investigator's<br />
HI<br />
0%<br />
(0/4)<br />
0%<br />
(0/4)<br />
0%<br />
(0/5)<br />
33%<br />
(1/3)<br />
20%<br />
(1/5)<br />
10%<br />
(2/21)<br />
<strong>Focus</strong> IgG<br />
ELISA<br />
25%<br />
(1/4)<br />
0%<br />
(0/4)<br />
0%<br />
(0/5)<br />
33%<br />
(1/3)<br />
40%<br />
(2/5)<br />
19%<br />
(4/21)<br />
STABILITY<br />
Released components were accelerated to at least an equivalent of 1 year at 2<br />
to 8°C by incubating at 37°C. The accelerated components were then tested<br />
according to the package insert in parallel with unaccelerated components.<br />
Components are considered stable if they meet the QC criteria specified in the<br />
package insert. The device passed each of the QC criteria.<br />
INTRA-ASSAY REPRODUCIBILITY<br />
Investigational Site 4 assessed intra-assay variation. Two strips each (8 wells<br />
per strip) were removed from six different plates, and the removed strips were<br />
re-assembled into a new plate (12 strips total, 96 wells total). The new plate<br />
was used to run an assay according to the package insert using Cut-off<br />
Calibrator as a sample. The device demonstrated the following intra-assay<br />
variation:<br />
Dengue Virus IgG DxSelect<br />
Page 2<br />
INTER-ASSAY REPRODUCIBILITY<br />
Investigational Site 4 assessed inter-assay variation. The controls and two<br />
different samples (one borderline and positive sera) were run once a week for<br />
six weeks. Each assay was run as described in the package insert. Mean,<br />
standard deviation (S.D.), coefficient of variation (C.V.), and % agreement for<br />
interpretation were calculated.<br />
<strong>Focus</strong> IgG ELISA Inter-assay Reproducibility<br />
Item n Mean S.D. C.V. % Agreement<br />
Cut-off Calibrator O.D. 6 0.342 0.055 16.1% NA<br />
Non-Detectable Control Index 6 0.023 0.011 49.4% 100% (6/6)<br />
Detectable Control Index 6 2.125 0.105 5.0% 100% (6/6)<br />
High Positive Control Index 6 4.990 0.396 7.9% 100% (6/6)<br />
(no longer available)<br />
Borderline Serum Index 6 0.965 0.148 15.4% 67% (4/6)<br />
Positive Serum Index 6 4.818 0.515 10.7% 100% (6/6)<br />
INTER-LOT REPRODUCIBILITY<br />
Investigational Site 7 assessed inter-lot variation. The controls and different<br />
samples (five positive and three negative sera) were run with two separate kit<br />
lots . The device demonstrated the following reproducibility.<br />
Sample #<br />
Index<br />
Interpretation<br />
Lot 1 Lot 2 % Agreement<br />
QC 1 0.412 0.166 100%<br />
QC 3 0.899 0.482 100%<br />
QC 4 1.294 1.596 100%<br />
QC 6 2.540 2.750 100%<br />
QC 7 3.726 4.174 100%<br />
QC 8 7.136 6.449 100%<br />
QC 9 13.521 10.504 100%<br />
QC 10 0.062 0.114 100%<br />
PC.EL1500G<br />
Rev.F<br />
Date written 11 Aug 2008<br />
<strong>Focus</strong> IgG ELISA Intra-assay Reproducibility<br />
Wells Mean O.D. Coefficient of<br />
Variation<br />
Strips<br />
Plate<br />
1 & 2 1 0.143 3.2%<br />
3 & 4 2 0.142 5.0%<br />
5 & 6 3 0.139 4.6%<br />
7 & 8 4 0.134 5.9%<br />
9 &10 5 0.136 4.8%<br />
11 & 12 6 0.143 5.8%<br />
All twelve strips, two strips<br />
from each plate (n = 96 wells)<br />
0.139 5.4%<br />
Cypress, California 90630 USA<br />
www.focusdx.com