Fick's Second Law
Fick's Second Law
Fick's Second Law
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
SHR §3.3.2<br />
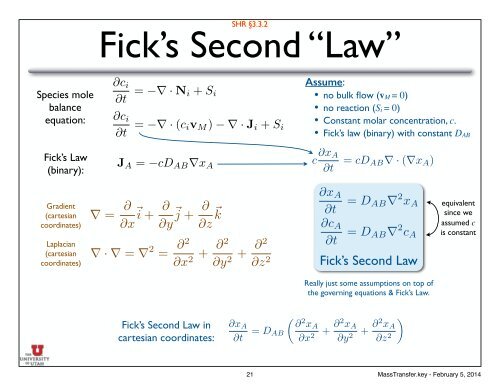
Fick’s <strong>Second</strong> “<strong>Law</strong>”<br />
Species mole<br />
balance<br />
equation:<br />
@c i<br />
@t = r · N i + S i<br />
@c i<br />
@t = r · (c iv M ) r· J i + S i<br />
Assume: <br />
• no bulk flow (vM = 0) <br />
• no reaction (Si = 0) <br />
• Constant molar concentration, c. <br />
• Fick’s law (binary) with constant DAB<br />
Fick’s <strong>Law</strong><br />
(binary):<br />
J A =<br />
cD AB rx A<br />
c @x A<br />
@t<br />
= cD AB r · (rx A )<br />
Gradient <br />
(cartesian<br />
coordinates)<br />
Laplacian <br />
(cartesian<br />
coordinates)<br />
r = @<br />
@x ~ i + @ @y ~ j + @ @z ~ k<br />
r · r = r 2 = @2<br />
@x 2 + @2<br />
@y 2 + @2<br />
@z 2<br />
@x A<br />
@t<br />
@c A<br />
@t<br />
= D AB r 2 x A equivalent<br />
since we<br />
= D AB r 2 assumed c<br />
c A is constant<br />
Fick’s <strong>Second</strong> <strong>Law</strong><br />
Really just some assumptions on top of<br />
the governing equations & Fick’s <strong>Law</strong>.<br />
Fick’s <strong>Second</strong> <strong>Law</strong> in<br />
cartesian coordinates:<br />
@x A<br />
@t<br />
= D AB<br />
✓ @ 2 x A<br />
@x 2<br />
+ @2 x A<br />
@y 2<br />
+ @2 x A<br />
@z 2<br />
◆<br />
21 MassTransfer.key - February 5, 2014
Example: Loschmidt Tube<br />
@c i<br />
@t = r · (c iv M ) r· J i + S i<br />
@x A<br />
@t<br />
What<br />
assumptions<br />
= D AB<br />
@ 2 x A<br />
xA+<br />
xA-<br />
z=<br />
z=0<br />
@z 2 t=0 t→∞<br />
How many intial & boundary conditions do<br />
we need What form should the BCs take<br />
z=-<br />
"<br />
(x A x A )<br />
(x A+ x A ) = 1<br />
2 + 1 ⇡<br />
1X<br />
k=0<br />
1<br />
⇣ m⇡z<br />
⌘ ✓ m 2<br />
m sin ⇡ 2 ◆ #<br />
exp Dt<br />
`<br />
`2<br />
1<br />
0.8<br />
time<br />
m = k + 1 2<br />
Reminder: solutions to PDEs are heavily<br />
influenced by initial and boundary conditions!<br />
Mole Fraction<br />
0.6<br />
0.4<br />
0.2<br />
time<br />
0<br />
−0.2 −0.15 −0.1 −0.05 0 0.05 0.1 0.15 0.2<br />
z<br />
22 MassTransfer.key - February 5, 2014
SHR §3.3.3<br />
Example: Ground Contamination<br />
A benzene spill occurs on the ground. Determine how long<br />
it will take for the contamination to diffuse 1 meter deep.<br />
@x A<br />
@t<br />
= D AB<br />
@ 2 x A<br />
@z 2<br />
c A = c Ao t =0<br />
c A = c As t>0, z=0<br />
c A =0 t>0, z!1<br />
✓<br />
c A c Ao<br />
=erfc<br />
c As c Ao<br />
t =<br />
apple<br />
z2<br />
4D AB<br />
Assumptions: <br />
• the spill is “large” in x and y<br />
directions (relative to z). <br />
• D AB =10 -8 cm 2 /s. <br />
• Fick’s second law holds. <br />
• Soil density is 2 g/cm 3 . <br />
• Benzene density is 0.8765 g/cm 3<br />
z<br />
2 p D AB t<br />
◆<br />
c Ao<br />
µg Benzene per kg soil<br />
✓ ◆ 2<br />
erfc 1 cA<br />
= 4.2 years<br />
c As c Ao<br />
4<br />
3<br />
2<br />
1<br />
x 10 8<br />
EPA suggests less than 0.3 μg<br />
benzene (A) per kg soil (B).<br />
c max<br />
A<br />
=3⇥ 10 7 gA/kgB ⇢ B<br />
M A<br />
=3⇥ 10 7 gA/kgB 2000kgB /m 3<br />
78 gA /molA<br />
=7.69 ⇥ 10 6 molA/m 3<br />
Each curve is a different<br />
time (in years!)<br />
1<br />
2<br />
4<br />
8<br />
16<br />
32<br />
64<br />
0<br />
0 0.5 1 1.5 2<br />
z (m)<br />
Disclaimer: there is actual flow (not just<br />
diffusion) in such a spill. Contamination<br />
could occur much faster...<br />
23 MassTransfer.key - February 5, 2014
Example: Steady-State Diffusion & Reaction<br />
A + B ! C<br />
S A = S B = S C = kc A c B<br />
0<br />
@c A<br />
@t<br />
@c B<br />
@t<br />
@c C<br />
@t<br />
= D A<br />
@ 2 c A<br />
@z 2<br />
= D B<br />
@ 2 c B<br />
@z 2<br />
= D C<br />
@ 2 c C<br />
@z 2<br />
+ S A<br />
+ S B<br />
+ S C<br />
cA,i-1<br />
cA,i cA,i+1<br />
Δz<br />
http://www.che.utah.edu/~sutherland/page_id=867<br />
24 MassTransfer.key - February 5, 2014
Length & Time Scales in Diffusion<br />
Fick’s <strong>Second</strong> <strong>Law</strong>:<br />
@x A<br />
@t<br />
= D AB<br />
@ 2 x A<br />
@z 2<br />
Let’s nondimensionalize this equation:<br />
nondimensional time: t ⇤ = t /⌧<br />
nondimensional length: z ⇤ = z /`<br />
nondimensional diffusivity: D ⇤ = D⌧ /`2<br />
If we choose and τ<br />
associated with diffusion,<br />
then we expect D*=1,<br />
⌧ = `2<br />
D<br />
` = p D⌧<br />
Characteristic length and time<br />
scales for diffusion!<br />
25 MassTransfer.key - February 5, 2014