April Journal-2009.p65 - Association of Biotechnology and Pharmacy
April Journal-2009.p65 - Association of Biotechnology and Pharmacy
April Journal-2009.p65 - Association of Biotechnology and Pharmacy
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Current Trends in <strong>Biotechnology</strong> <strong>and</strong> <strong>Pharmacy</strong><br />
Vol. 3 (2) 113-127, <strong>April</strong> 2009. ISSN 0973-8916<br />
pr<strong>of</strong>essional to distribute <strong>and</strong> oversee vaccinations<br />
but these conditions are not required for edible<br />
vaccines. The goal is to give people in developing<br />
countries the genetically engineered seeds that<br />
will sprout edible vaccines. “Every culture on this<br />
planet raises food” explains Hein. “This can<br />
provide developing countries with a stable vaccine<br />
source because it will be genetically coded into<br />
the food”. Using recombinant DNA technology,<br />
researchers can now isolate the genes—called<br />
antigens—that mobilize our natural defences. But<br />
impregnating plants with these antigens requires<br />
an impressive bit <strong>of</strong> molecular legerdemain. At<br />
Scrips Research Institute, for instance, the antigen<br />
116<br />
is snipped <strong>of</strong>f the deadly cholera pathogen. Then<br />
it is inserted into the cells <strong>of</strong> a bacterium that<br />
causes a plant disease called crown gall. The<br />
alfalfa plants are infected with these transgenic<br />
crown gall organisms, which can penetrate the<br />
plant’s cell walls. The plant cells containing the<br />
foreign genes are then cultured in a Petri dish<br />
until they are mature enough to be transplanted.<br />
The next step is to test the potency <strong>of</strong> the antigens<br />
in plants raised in the field, outside <strong>of</strong> the cloistered<br />
laboratory. “We’ve just harvested this crop <strong>of</strong><br />
alfalfa” says Hein, who’s in the midst <strong>of</strong> measuring<br />
its antigen level <strong>and</strong> feed this transgenic grain to<br />
mice (64, 39 & 40, 24, 1,2 & 3, 48, 8, 21, 4). .<br />
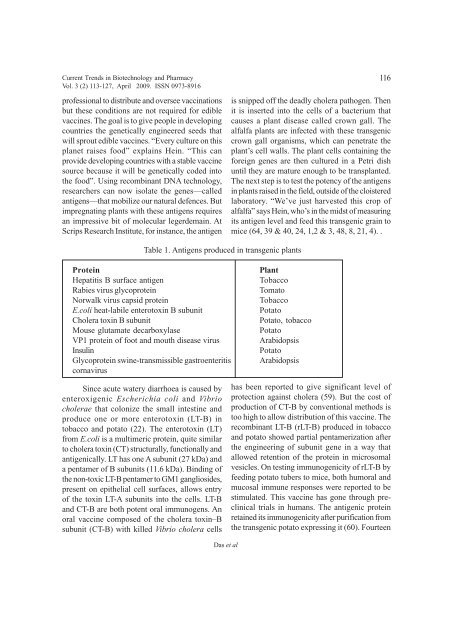
Table 1. Antigens produced in transgenic plants<br />
Protein<br />
Hepatitis B surface antigen<br />
Rabies virus glycoprotein<br />
Norwalk virus capsid protein<br />
E.coli heat-labile enterotoxin B subunit<br />
Cholera toxin B subunit<br />
Mouse glutamate decarboxylase<br />
VP1 protein <strong>of</strong> foot <strong>and</strong> mouth disease virus<br />
Insulin<br />
Glycoprotein swine-transmissible gastroenteritis<br />
cornavirus<br />
Plant<br />
Tobacco<br />
Tomato<br />
Tobacco<br />
Potato<br />
Potato, tobacco<br />
Potato<br />
Arabidopsis<br />
Potato<br />
Arabidopsis<br />
Since acute watery diarrhoea is caused by<br />
enteroxigenic Escherichia coli <strong>and</strong> Vibrio<br />
cholerae that colonize the small intestine <strong>and</strong><br />
produce one or more enterotoxin (LT-B) in<br />
tobacco <strong>and</strong> potato (22). The enterotoxin (LT)<br />
from E.coli is a multimeric protein, quite similar<br />
to cholera toxin (CT) structurally, functionally <strong>and</strong><br />
antigenically. LT has one A subunit (27 kDa) <strong>and</strong><br />
a pentamer <strong>of</strong> B subunits (11.6 kDa). Binding <strong>of</strong><br />
the non-toxic LT-B pentamer to GM1 gangliosides,<br />
present on epithelial cell surfaces, allows entry<br />
<strong>of</strong> the toxin LT-A subunits into the cells. LT-B<br />
<strong>and</strong> CT-B are both potent oral immunogens. An<br />
oral vaccine composed <strong>of</strong> the cholera toxin–B<br />
subunit (CT-B) with killed Vibrio cholera cells<br />
has been reported to give significant level <strong>of</strong><br />
protection against cholera (59). But the cost <strong>of</strong><br />
production <strong>of</strong> CT-B by conventional methods is<br />
too high to allow distribution <strong>of</strong> this vaccine. The<br />
recombinant LT-B (rLT-B) produced in tobacco<br />
<strong>and</strong> potato showed partial pentamerization after<br />
the engineering <strong>of</strong> subunit gene in a way that<br />
allowed retention <strong>of</strong> the protein in microsomal<br />
vesicles. On testing immunogenicity <strong>of</strong> rLT-B by<br />
feeding potato tubers to mice, both humoral <strong>and</strong><br />
mucosal immune responses were reported to be<br />
stimulated. This vaccine has gone through preclinical<br />
trials in humans. The antigenic protein<br />
retained its immunogenicity after purification from<br />
the transgenic potato expressing it (60). Fourteen<br />
Das et al