December 2012 - Monthly Update, Focus Diagnostics, Incorporated
December 2012 - Monthly Update, Focus Diagnostics, Incorporated
December 2012 - Monthly Update, Focus Diagnostics, Incorporated
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>December</strong> <strong>2012</strong> - <strong>Monthly</strong> <strong>Update</strong>, <strong>Focus</strong> <strong>Diagnostics</strong>, <strong>Incorporated</strong><br />
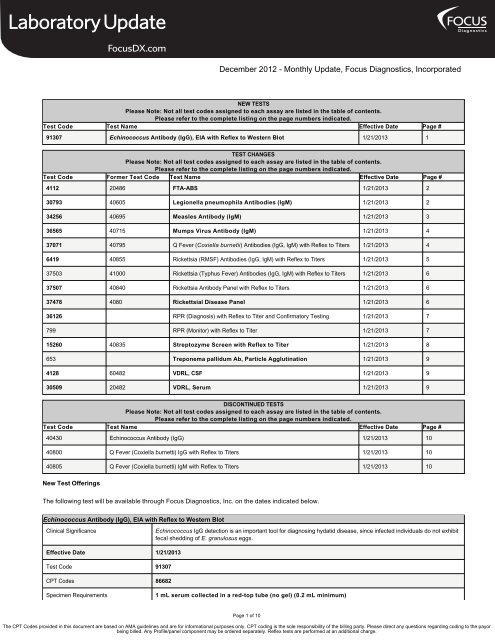
NEW TESTS<br />
Please Note: Not all test codes assigned to each assay are listed in the table of contents.<br />
Please refer to the complete listing on the page numbers indicated.<br />
Test Code Test Name Effective Date Page #<br />
91307 Echinococcus Antibody (IgG), EIA with Reflex to Western Blot 1/21/2013 1<br />
TEST CHANGES<br />
Please Note: Not all test codes assigned to each assay are listed in the table of contents.<br />
Please refer to the complete listing on the page numbers indicated.<br />
Test Code Former Test Code Test Name Effective Date Page #<br />
4112 20486 FTA-ABS 1/21/2013 2<br />
30793 40605 Legionella pneumophila Antibodies (IgM) 1/21/2013 2<br />
34256 40695 Measles Antibody (IgM) 1/21/2013 3<br />
36565 40715 Mumps Virus Antibody (IgM) 1/21/2013 4<br />
37071 40795 Q Fever (Coxiella burnetii) Antibodies (IgG, IgM) with Reflex to Titers 1/21/2013 4<br />
6419 40855 Rickettsia (RMSF) Antibodies (IgG, IgM) with Reflex to Titers 1/21/2013 5<br />
37503 41000 Rickettsia (Typhus Fever) Antibodies (IgG, IgM) with Reflex to Titers 1/21/2013 6<br />
37507 40840 Rickettsia Antibody Panel with Reflex to Titers 1/21/2013 6<br />
37478 4080 Rickettsial Disease Panel 1/21/2013 6<br />
36126 RPR (Diagnosis) with Reflex to Titer and Confirmatory Testing 1/21/2013 7<br />
799 RPR (Monitor) with Reflex to Titer 1/21/2013 7<br />
15260 40835 Streptozyme Screen with Reflex to Titer 1/21/2013 8<br />
653 Treponema pallidum Ab, Particle Agglutination 1/21/2013 9<br />
4128 60482 VDRL, CSF 1/21/2013 9<br />
30509 20482 VDRL, Serum 1/21/2013 9<br />
DISCONTINUED TESTS<br />
Please Note: Not all test codes assigned to each assay are listed in the table of contents.<br />
Please refer to the complete listing on the page numbers indicated.<br />
Test Code Test Name Effective Date Page #<br />
40430 Echinococcus Antibody (IgG) 1/21/2013 10<br />
40800 Q Fever (Coxiella burnetti) IgG with Reflex to Titers 1/21/2013 10<br />
40805 Q Fever (Coxiella burnetti) IgM with Reflex to Titers 1/21/2013 10<br />
New Test Offerings<br />
The following test will be available through <strong>Focus</strong> <strong>Diagnostics</strong>, Inc. on the dates indicated below.<br />
Echinococcus Antibody (IgG), EIA with Reflex to Western Blot<br />
Clinical Significance<br />
Echinococcus IgG detection is an important tool for diagnosing hydatid disease, since infected individuals do not exhibit<br />
fecal shedding of E. granulosus eggs.<br />
Effective Date 1/21/2013<br />
Test Code 91307<br />
CPT Codes 86682<br />
Specimen Requirements<br />
1 mL serum collected in a red-top tube (no gel) (0.2 mL minimum)<br />
Page 1 of 10<br />
The CPT Codes provided in this document are based on AMA guidelines and are for informational purposes only. CPT coding is the sole responsibility of the billing party. Please direct any questions regarding coding to the payor<br />
being billed. Any Profile/panel component may be ordered separately. Reflex tests are performed at an additional charge.
<strong>December</strong> <strong>2012</strong> - <strong>Monthly</strong> <strong>Update</strong>, <strong>Focus</strong> <strong>Diagnostics</strong>, <strong>Incorporated</strong><br />
Transport Temperature<br />
Specimen Stability<br />
Set-up/Analytic Time<br />
Reference Range<br />
Always Message<br />
Room temperature<br />
Room temperature: 7 days<br />
Refrigerated: 14 days<br />
Frozen: 30 days<br />
Set up: Tues, Fri; Report available: 1-5 days<br />
Negative<br />
REFERENCE RANGE: NEGATIVE<br />
Detection of serum antibodies to Echinococcus plays an important role in the diagnosis of hydatid<br />
disease, since infected individuals do not exhibit fecal shedding of Echinococcus eggs. Serum antibodies<br />
may remain detectable for years after cyst removal. Serologic crossreactivity between Echinococcus and<br />
Cysticercus may occur.<br />
Methodology<br />
CPU Mappings<br />
Immunoassay<br />
Result Code<br />
Result Name<br />
913071 Echinococcus IgG, EIA<br />
This is a true reflex. Please build the unit codes below separately.Non-Orderable Reflex 913071<br />
Echinococcus Antibody (IgG), Western Blot<br />
Result Code<br />
Result Name<br />
9130710 Echinococcus IgG, WB<br />
Additional Information<br />
If the Echinococcus IgG result is Positive, then the Echinococcus Ab (IgG), Western Blot will be performed<br />
at an additional charge (CPT code: 86682).<br />
Test Changes<br />
FTA-ABS<br />
Effective Date 1/21/2013<br />
Former Test Name<br />
Treponema pallidum Antibody, IFA (Serum)<br />
Former Test Code 20486<br />
Test Code 4112<br />
Specimen Requirements<br />
Reject Criteria<br />
Transport Temperature<br />
Specimen Stability<br />
Set-up/Analytic Time<br />
Always Message<br />
CPU Mappings<br />
1 mL serum (0.2 mL minimum)<br />
Gross hemolysis; gross lipemia<br />
Room temperature<br />
Room temperature: 7 days<br />
Refrigerated: 14 days<br />
Frozen: 30 days<br />
Set up: Mon-Fri; Report available; 1-4 days<br />
Remove current Always Message<br />
Result Code<br />
Result Name<br />
40010300 FTA-ABS<br />
Page 2 of 10
<strong>December</strong> <strong>2012</strong> - <strong>Monthly</strong> <strong>Update</strong>, <strong>Focus</strong> <strong>Diagnostics</strong>, <strong>Incorporated</strong><br />
Legionella pneumophila Antibodies (IgM)<br />
Effective Date 1/21/2013<br />
Former Test Name<br />
Legionella pneumophila Antibody (IgM), IFA<br />
Former Test Code 40605<br />
Test Code 30793<br />
CPT Codes<br />
Transport Temperature<br />
Specimen Stability<br />
Set-up/Analytic Time<br />
86713 (x2)<br />
Room temperature<br />
Room temperature: 7 days<br />
Refrigerated: 14 days<br />
Frozen: 30 days<br />
Set up: Mon-Fri; Report available: 1-4 days<br />
Reference Range Reference Range:
<strong>December</strong> <strong>2012</strong> - <strong>Monthly</strong> <strong>Update</strong>, <strong>Focus</strong> <strong>Diagnostics</strong>, <strong>Incorporated</strong><br />
Assay Category ASR Class 1<br />
and convalescent sera. However, detection of IgM antibody in a single specimen may indicate acute<br />
disease. Correct interpretation of serologic data depends upon the proper timing of specimen collection<br />
in relation to rash onset.<br />
This timing is particularly important for interpreting negative IgM results, since IgM antibody peaks<br />
approximately 10 days after rash onset and is usually undetectable 30 days after rash onset.<br />
This assay was developed and its performance characteristics have been determined by <strong>Focus</strong><br />
<strong>Diagnostics</strong>. It has not been cleared or approved by the U.S. Food and Drug Administration. The FDA has<br />
determined that such clearance or approval is not necessary. Performance characteristics refer to the<br />
analytical performance of the test.<br />
CPU Mappings<br />
Result Code<br />
Result Name<br />
70035550 Measles Antibody, IgM<br />
Mumps Virus Antibody (IgM)<br />
Effective Date 1/21/2013<br />
Former Test Name<br />
MUMPS IgM ANTIBODY, IFA (SERUM)<br />
Former Test Code 40715<br />
Test Code 36565<br />
Specimen Requirements<br />
Reject Criteria<br />
Transport Temperature<br />
Specimen Stability<br />
Set-up/Analytic Time<br />
CPU Mappings<br />
1 mL serum (0.2 mL minimum)<br />
Gross Hemolysis, gross lipemia<br />
Room temperature<br />
Room temperature: 7 days<br />
Refrigerated: 14 days<br />
Frozen: 30 days<br />
Set up: Mon-Fri; Report available: 1-4 days<br />
Result Code<br />
Result Name<br />
70056200 Mumps Virus Antibody IgM<br />
Q Fever (Coxiella burnetii) Antibodies (IgG, IgM) with Reflex to Titers<br />
Effective Date 1/21/2013<br />
Former Test Code 40795<br />
Test Code 37071<br />
Set-up/Analytic Time<br />
CPU Mappings<br />
Set up: Mon- Fri; Report: 1-4 days<br />
Non orderable TC 38505 Q Fever IgG Phase I Screen<br />
Result Code<br />
Result Name<br />
85995406 Q Fever IgG Phase I Scr<br />
Non orderable TC 38507 Q Fever IgG Phase II Screen<br />
Result Code<br />
Result Name<br />
85995408 Q Fever IgG Phase II Scr<br />
Non orderable TC 38509 Q Fever IgM Phase I Screen<br />
Result Code<br />
Result Name<br />
85995410 Q Fever IgM Phase I Scr<br />
Page 4 of 10
<strong>December</strong> <strong>2012</strong> - <strong>Monthly</strong> <strong>Update</strong>, <strong>Focus</strong> <strong>Diagnostics</strong>, <strong>Incorporated</strong><br />
Non orderable TC 38511 Q Fever IgM Phase II Screen<br />
Result Code<br />
Result Name<br />
85995412 Q Fever IgM Phase II Scr<br />
This is a true reflex. Please build the unit codes below separately. Non Orderable Reflex 38506 Q Fever<br />
IgG Phase I Titer<br />
Result Code<br />
Result Name<br />
85995407 Q Fever IgG Phase I Titer<br />
Non Orderable Reflex 38508 Q Fever IgG Phase II Titer<br />
Result Code<br />
Result Name<br />
85995409 Q Fever IgG Phase II Titer<br />
Non Orderable Reflex 38510 Q Fever IgM Phase I Titer<br />
Result Code<br />
Result Name<br />
85995411 Q Fever IgM Phase I Titer<br />
Non Orderable Reflex 38512 Q Fever IgM Phase II Titer<br />
Result Code<br />
Result Name<br />
85995413 Q Fever IgM Phase II Titer<br />
Additional Information<br />
If the Q Fever screen is Positive, IgG or IgM Phase I or Phase II then the appropriate Titer will be performed at an<br />
additional charge (CPT code(s): 86638 per titer).<br />
Rickettsia (RMSF) Antibodies (IgG, IgM) with Reflex to Titers<br />
Effective Date 1/21/2013<br />
Former Test Code 40855<br />
Test Code 6419<br />
Reject Criteria<br />
Set-up/Analytic Time<br />
Always Message<br />
CPU Mappings<br />
Gross hemolysis; gross lipemia<br />
Set up; Mon-Fri; Report available: 1-4 days<br />
Remove current Always Message<br />
6419 Rickettsia (RMSF) Antibodies (IgG, IgM) with Reflex to Titers<br />
Result Code<br />
Result Name<br />
70025900 RMSF IgG<br />
70035700 RMSF IgM<br />
This is a true reflex. Please build the unit codes below separately. Non-Orderable Reflex 64192 RMSF<br />
Antibody Titer, IgG<br />
Result Code<br />
Result Name<br />
70025906 RMSF IgG Titer<br />
This is a true reflex. Please build the unit codes below separately. Non-Orderable Reflex 64193 RMSF<br />
Antibody Titer, IgM<br />
Result Code<br />
Result Name<br />
70035706 RMSF IgM Titer<br />
Page 5 of 10
<strong>December</strong> <strong>2012</strong> - <strong>Monthly</strong> <strong>Update</strong>, <strong>Focus</strong> <strong>Diagnostics</strong>, <strong>Incorporated</strong><br />
Additional Information<br />
If the Rickettsia (RMSF) screen is Detected, IgG or IgM, then the appropriate Titer will be performed at an additional<br />
charge (CPT code(s) 86757 per titer).<br />
Rickettsia (Typhus Fever) Antibodies (IgG, IgM) with Reflex to Titers<br />
Effective Date 1/21/2013<br />
Former Test Code 41000<br />
Test Code 37503<br />
Reject Criteria<br />
Set-up/Analytic Time<br />
Always Message<br />
CPU Mappings<br />
Gross hemolysis; gross lipemia<br />
Set up: Mon-Fri; Report available: 1-4 days<br />
Remove current Always Message<br />
37503 Rickettsia (Typhus Fever) Antibodies (IgG, IgM) with Reflex to Titers<br />
Reporting Title: RICKETT (TYPHUS) AB W/RFL<br />
Result Code<br />
Result Name<br />
70026000 R. typhi IgG<br />
70026040 R. typhi IgM<br />
This is a true reflex. Please build the unit codes below separately. Non-Orderable Reflex 375032 R. Typhi<br />
IgG Titer<br />
Result Code<br />
Result Name<br />
70026021 R. typhi IgG Titer<br />
This is a true reflex. Please build the unit codes below separately. Non-Orderable Reflex 375033 R. Typhi<br />
IgM Titer<br />
Result Code<br />
Result Name<br />
70026071 R. typhi IgM Titer<br />
Additional Information<br />
If the Rickettsia (Typhus Fever) screen is Detected, IgG or IgM, then the appropriate Titer will be performed at an<br />
additional charge (CPT code(s) 86757 per titer)<br />
Rickettsia Antibody Panel with Reflex to Titers<br />
Effective Date 1/21/2013<br />
Former Test Code 40840<br />
Test Code 37507<br />
Reject Criteria<br />
Set-up/Analytic Time<br />
Always Message<br />
CPU Mappings<br />
Additional Information<br />
Gross hemolysis; gross lipemia<br />
Set up: Mon-Fri; Report available: 1-4 days<br />
Remove current Always Message<br />
See individual mapping for test codes:<br />
6419 Rickettsia (RMSF) Antibodies (IgG, IgM) with Reflex to Titers<br />
37503 Rickettsia (Typhus Fever) Antibodies (IgG, IgM) with Reflex to Titers<br />
If the Rickettsia (RMSF) screen is Detected, IgG or IgM, then the appropriate Titer will be performed at an additional<br />
charge (CPT code(s) 86757 per titer).<br />
If the Rickettsia (Typhus Fever) screen is Detected, IgG or IgM, then the appropriate Titer will be performed at an<br />
additional charge (CPT code(s) 86757 per titer)<br />
Page 6 of 10
<strong>December</strong> <strong>2012</strong> - <strong>Monthly</strong> <strong>Update</strong>, <strong>Focus</strong> <strong>Diagnostics</strong>, <strong>Incorporated</strong><br />
Rickettsial Disease Panel<br />
Effective Date 1/21/2013<br />
Former Test Name<br />
RICKETTSIA AND COXIELLA BURNETII ANTIBODY PANEL<br />
Former Test Code 4080<br />
Test Code 37478<br />
Specimen Requirements<br />
Reject Criteria<br />
Set-up/Analytic Time<br />
Always Message<br />
CPU Mappings<br />
Additional Information<br />
2 mL serum (0.5 mL minimum)<br />
Gross hemolysis; gross lipemia<br />
Set up: Mon-Fri; Report available: 1-4 days<br />
Remove current Always Message<br />
See individual mapping for test codes:<br />
6419 Rickettsia (RMSF) Antibodies (IgG, IgM) with Reflex to Titers<br />
37503 Rickettsia (Typhus Fever) Antibodies (IgG, IgM) with Reflex to Titers<br />
Non orderable TC 38505 - Q Fever IgG Phase I Screen<br />
Non orderable TC 38507 - Q Fever IgG Phase II Screen<br />
Non orderable TC 38509 - Q Fever IgM Phase I Screen<br />
Non orderable TC 38511 - Q Fever IgM Phase II Screen<br />
If the Rickettsia (RMSF) screen is Detected, IgG or IgM, then the appropriate Titer will be performed at an additional<br />
charge (CPT code(s) 86757 per titer).<br />
If the Rickettsia (Typhus Fever) screen is Detected, IgG or IgM, then the appropriate Titer will be performed at an<br />
additional charge (CPT code(s) 86757 per titer)<br />
If the Q Fever screen is Positive, IgG or IgM Phase I or Phase II then the appropriate Titer will be performed at an<br />
additional charge (CPT code(s) 86638 per titer).<br />
RPR (Diagnosis) with Reflex to Titer and Confirmatory Testing<br />
Effective Date 1/21/2013<br />
Test Code 36126<br />
Reject Criteria<br />
Transport Temperature<br />
Set-up/Analytic Time<br />
Always Message<br />
Methodology<br />
CPU Mappings<br />
Gross hemolysis; gross lipemia<br />
Room temperature<br />
Set Up; Tues-Sat; Report available: 1-5 days<br />
The RPR is a non-treponemal-specific test; therefore, a treponemal-specific confirmatory test should be<br />
performed unless prior syphilis infection has been documented for this patient.<br />
Flocculation<br />
36126 RPR (Diagnosis) with Reflex to Titer and Confirmatory Testing<br />
Reporting Title: RPR (DIAG) W/RFL TTR CONF<br />
Result Code<br />
Result Name<br />
86007407 RPR Screen<br />
This is a true reflex. Please build the unit codes below separately. Non-Orderable Reflex 361262 RPR<br />
Titer<br />
Result Code<br />
Result Name<br />
86007408 RPR Titer<br />
This is a true reflex. Please build the unit codes below separately. Non-Orderable Reflex 361263 FTA,<br />
Confirmatory Testing<br />
Result Code<br />
Result Name<br />
40010300 FTA-ABS<br />
Page 7 of 10
<strong>December</strong> <strong>2012</strong> - <strong>Monthly</strong> <strong>Update</strong>, <strong>Focus</strong> <strong>Diagnostics</strong>, <strong>Incorporated</strong><br />
Additional Information<br />
If the RPR Screen is “Reactive” then the RPR Titer and FTA Confirmatory Testing will be performed at<br />
additional charges (CPT code(s) 86593 and 86780).<br />
RPR (Monitor) with Reflex to Titer<br />
Effective Date 1/21/2013<br />
Test Code 799<br />
Reject Criteria<br />
Transport Temperature<br />
Set-up/Analytic Time<br />
Always Message<br />
Methodology<br />
CPU Mappings<br />
Gross hemolysis; gross lipemia<br />
Room temperature<br />
Set up: Tues-Sat; Report available: 1-5 days<br />
The RPR is a non-treponemal-specific test; therefore, a treponemal-specific confirmatory test should be<br />
performed unless prior syphilis infection has been documented for this patient.<br />
Flocculation<br />
799 RPR (Monitor) with Reflex to Titer<br />
Reporting Title: RPR (MONITOR) W/RFL TITER<br />
Result Code<br />
Result Name<br />
86007407 RPR Screen<br />
This is a true reflex. Please build the unit codes below separately. Non-Orderable Reflex 7992 RPR Titer<br />
Result Code<br />
Result Name<br />
86007408 RPR Titer<br />
Additional Information<br />
If the RPR Screen is Reactive, then the RPR Titer will be performed at an additional charge (CPT code<br />
86593).<br />
Streptozyme Screen with Reflex to Titer<br />
Effective Date 1/21/2013<br />
Former Test Code 40835<br />
Test Code 15260<br />
Specimen Requirements<br />
Set-up/Analytic Time<br />
Reference Range<br />
Methodology<br />
CPU Mappings<br />
1 mL serum (0.2 mL minimum)<br />
Plasma is no longer acceptable<br />
Remove current collection instructions<br />
Set up: Mon-Fri; Report available: 1-4 days<br />
Screen: Negative<br />
Titer: < 1:100 titer<br />
Slide Hemagglutination<br />
15260 Streptozyme Screen with Reflex to Titer<br />
Reporting Title: STREPTOZYME SCR W/RFL TITER<br />
Result Code<br />
Result Name<br />
40039405 Streptozyme Screen<br />
This is a true reflex. Please build the unit codes below separately. Non-Orderable Reflex 152602<br />
Streptozyme Titer<br />
Result Code<br />
Result Name<br />
Page 8 of 10
<strong>December</strong> <strong>2012</strong> - <strong>Monthly</strong> <strong>Update</strong>, <strong>Focus</strong> <strong>Diagnostics</strong>, <strong>Incorporated</strong><br />
40039410<br />
Streptozyme Titer<br />
Additional Information<br />
If the Streptozyme Screen is “Positive” then the Streptozyme Titer will be performed at an additional<br />
charge (CPT code 86406).<br />
Treponema pallidum Ab, Particle Agglutination<br />
Effective Date 1/21/2013<br />
Test Code 653<br />
Reject Criteria<br />
Set-up/Analytic Time<br />
CPU Mappings<br />
Hemolysis; lipemia; plasma; CSF<br />
Set up: Tue, Fri; Report available: 1-5 days<br />
Reporting Title: TREPONEMA PALLIDUM AB<br />
Result Code<br />
Result Name<br />
86001191 T pallidum Ab (TP-PA)<br />
VDRL, CSF<br />
Effective Date 1/21/2013<br />
Former Test Name<br />
VDRL, (CSF)<br />
Former Test Code 60482<br />
Test Code 4128<br />
Specimen Requirements<br />
Reject Criteria<br />
Transport Temperature<br />
Specimen Stability<br />
Set-up/Analytic Time<br />
Always Message<br />
Methodology<br />
CPU Mappings<br />
1 mL CSF (0.2 mL minimum)<br />
Hemolysis; lipemia<br />
Refrigerated<br />
Room temperature: 4 days<br />
Refrigerated; 14 days<br />
Frozen: 6 months<br />
Set up: Mon-Fri; Report available: 1-4 days<br />
Remove current Always Message<br />
Slide Micro-Flocculation<br />
Result Code<br />
Result Name<br />
40010700 VDRL, CSF<br />
VDRL, Serum<br />
Effective Date 1/21/2013<br />
Former Test Name<br />
VDRL, (Serum)<br />
Former Test Code 20482<br />
Test Code 30509<br />
Specimen Requirements<br />
Set-up/Analytic Time<br />
Always Message<br />
1 mL serum (0.5 mL minimum)<br />
Set up: Mon-Fri; Report available: 1-4 days<br />
The VDRL is a non-treponemal-specific test; therefore, a treponemal-specific confirmatory test should<br />
be performed unless prior syphilis infection has been documented for this patient.<br />
Page 9 of 10
<strong>December</strong> <strong>2012</strong> - <strong>Monthly</strong> <strong>Update</strong>, <strong>Focus</strong> <strong>Diagnostics</strong>, <strong>Incorporated</strong><br />
Methodology<br />
CPU Mappings<br />
Slide Micro-Flocculation<br />
Result Code<br />
Result Name<br />
40015710 VDRL, Serum<br />
Discontinued Tests<br />
Echinococcus Antibody (IgG)<br />
Effective Date 1/21/2013<br />
Test Code 40430<br />
Additional Information<br />
The recommended alternative is:<br />
●<br />
91307 Echinococcus Antibody (IgG), EIA with Reflex to Western Blot<br />
Q Fever (Coxiella burnetti) IgG with Reflex to Titers<br />
Effective Date 1/21/2013<br />
Test Code 40800<br />
Additional Information<br />
The recommended alternative is:<br />
●<br />
37071 Q Fever (Coxiella burnetii) Antibodies (IgG, IgM) with Reflex to Titers<br />
Q Fever (Coxiella burnetti) IgM with Reflex to Titers<br />
Effective Date 1/21/2013<br />
Test Code 40805<br />
Additional Information<br />
The recommended alternative is:<br />
●<br />
37071 Q Fever (Coxiella burnetii) Antibodies (IgG, IgM) with Reflex to Titers<br />
Page 10 of 10<br />
Quest, Quest <strong>Diagnostics</strong>, the associated logo, Nichols Institute and all associated<br />
Quest <strong>Diagnostics</strong> marks are the trademarks of Quest <strong>Diagnostics</strong>. ©<strong>2012</strong> Quest <strong>Diagnostics</strong> <strong>Incorporated</strong>. All rights reserved. www.questdiagnostics.com