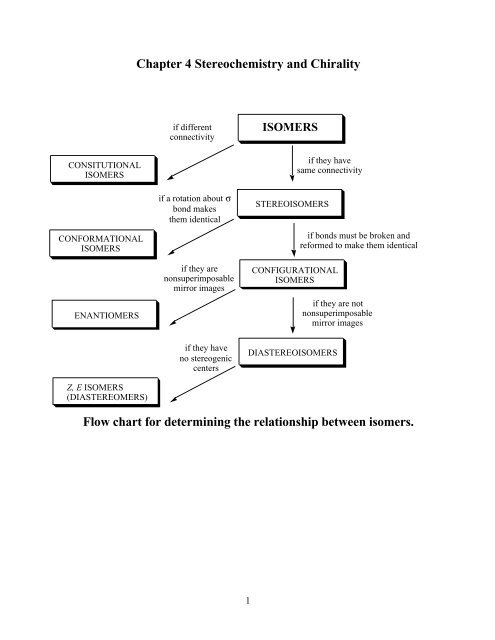
Chapter 4 Stereochemistry and Chirality Flow chart for determining ...
Chapter 4 Stereochemistry and Chirality Flow chart for determining ...
Chapter 4 Stereochemistry and Chirality Flow chart for determining ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Chapter</strong> 4 <strong>Stereochemistry</strong> <strong>and</strong> <strong>Chirality</strong><br />
if different<br />
connectivity<br />
ISOMERS<br />
CONSITUTIONAL<br />
ISOMERS<br />
if they have<br />
same connectivity<br />
CONFORMATIONAL<br />
ISOMERS<br />
ENANTIOMERS<br />
if a rotation about σ<br />
bond makes<br />
them identical<br />
if they are<br />
nonsuperimposable<br />
mirror images<br />
STEREOISOMERS<br />
CONFIGURATIONAL<br />
ISOMERS<br />
if bonds must be broken <strong>and</strong><br />
re<strong>for</strong>med to make them identical<br />
if they are not<br />
nonsuperimposable<br />
mirror images<br />
Z, E ISOMERS<br />
(DIASTEREOMERS)<br />
if they have<br />
no stereogenic<br />
centers<br />
DIASTEREOISOMERS<br />
<strong>Flow</strong> <strong>chart</strong> <strong>for</strong> <strong>determining</strong> the relationship between isomers.<br />
1
Symmetry Elements<br />
A mirror plane in Cartesian coordinates that includes the y <strong>and</strong> z axis<br />
means that <strong>for</strong> every point {x,y,z} there is a corresponding point {–x,y,z}. Ifa<br />
molecule has a mirror plane, then <strong>for</strong> every atom on one side of the plane there<br />
is an equivalent atom that is the mirror equivalent on the opposite side of the<br />
plane. When two identical groups are on one carbon, there is an internal mirror<br />
plane passing through the molecule.<br />
H<br />
H<br />
H<br />
O<br />
H<br />
H<br />
H<br />
H<br />
H<br />
2
Symmetry Elements (continued)<br />
An inversion center takes every point {x,y,z} to an equivalent point<br />
{–x,–y,–z}:<br />
Br<br />
CH 3<br />
H<br />
H<br />
H<br />
H<br />
H 3 C<br />
Br<br />
3
<strong>Chirality</strong><br />
The word "chirality" (from the Greek) refers to the property of "h<strong>and</strong>edness".<br />
To first approximation your right <strong>and</strong> left h<strong>and</strong>s are mirror images that cannot<br />
be superimposed on top of each other.<br />
4
<strong>Chirality</strong> (continued)<br />
Molecules that lack both a mirror plane <strong>and</strong> an inversion center can have nonsuperimposable<br />
mirror images <strong>and</strong> are said to be chiral. A chiral center<br />
usually is a tetrahedral carbon with four different groups attached to it. Chiral<br />
carbons are also referred to as stereocenters, stereogenic centers, or<br />
asymmmetric centers.<br />
1-Bromo-1-chloroethane is chiral:<br />
1<br />
H<br />
H<br />
4<br />
3<br />
2<br />
H 3 C<br />
Br<br />
Cl<br />
Br<br />
Cl<br />
CH 3<br />
H<br />
H<br />
H 3 C<br />
Br<br />
Cl<br />
H 3 C<br />
Cl<br />
Br<br />
H<br />
CH 3<br />
H 3 C<br />
Br<br />
Cl<br />
H<br />
Br<br />
Cl<br />
5
<strong>Chirality</strong> (continued)<br />
Any molecule that has an internal mirror plane is achiral.<br />
Cl<br />
Br<br />
H<br />
H<br />
H 3 C CH 3<br />
Cl<br />
Cl Br Br<br />
H 3 C CH 3<br />
Cl<br />
Cl<br />
Br Br<br />
A molecule that has an inversion center is achiral.<br />
Br<br />
CH 3<br />
H 3 C<br />
Br<br />
H<br />
H<br />
H<br />
H<br />
H<br />
H<br />
H<br />
H<br />
H 3 C<br />
Br<br />
Br<br />
rotate 90°<br />
CH 3<br />
Br<br />
CH 3<br />
H<br />
CH 3<br />
H<br />
H<br />
H<br />
H<br />
H<br />
Br<br />
Br<br />
H<br />
H 3<br />
C<br />
Br<br />
H 3 C<br />
H<br />
rotate 180°<br />
6
<strong>Chirality</strong> (continued)<br />
Further examples of achiral <strong>and</strong> chiral molecules:<br />
HO<br />
OH<br />
H<br />
H<br />
Br<br />
Br<br />
H<br />
Cl<br />
Cl<br />
H<br />
achiral achiral achiral<br />
HO<br />
OH<br />
Cl<br />
H CH 3<br />
Br<br />
H 3 C<br />
H<br />
Cl<br />
Cl<br />
H<br />
Br<br />
chiral chiral chiral<br />
7
Stereoisomers: Enantiomers<br />
Molecules that are mirror images but are not superimposable are called<br />
enantiomers. Enantiomers are stereoisomers. That is, they are isomers that<br />
have the same connectivity, but have groups that occupy different regions of<br />
space.<br />
Enantiomers have the same physical properties (e.g. melting points,<br />
boiling points, etc) but differ in the way they interact with other chiral objects<br />
(e.g. polarized light or different chiral molecules).<br />
It is possible to have a molecule with no chiral center that is chiral.<br />
Consider 1,3-dimethylallene, shown below:<br />
H 3 C<br />
•<br />
H<br />
H<br />
•<br />
CH 3<br />
H<br />
CH 3<br />
H 3 C<br />
H<br />
H 3 C<br />
H<br />
•<br />
H<br />
CH 3<br />
H 3 C<br />
H<br />
•<br />
H<br />
CH 3<br />
H 3 C<br />
•<br />
H<br />
H<br />
•<br />
H<br />
H<br />
CH 3<br />
H 3 C<br />
CH 3<br />
8
Stereoisomers: Diastereomers<br />
Stereoisomers that are not enantiomers are called diastereomers.<br />
H 3 C<br />
C 2 H 5<br />
Cl<br />
Br<br />
Br Cl<br />
H 3 C<br />
C 2 H 5<br />
Br<br />
Br<br />
Cl Cl<br />
For a molecule with multiple chiral centers, the number of possible<br />
stereoisomers is given by:<br />
x = 2 n<br />
where x is the number of possible isomers <strong>and</strong> n is the number of chiral centers.<br />
Thus, <strong>for</strong> molecules with two chiral centers there are four possible<br />
stereoisomers. (e.g. cholesterol, which has eight stereogenic centers, has 256<br />
possible stereoisomers!! )<br />
9
Stereoisomers: Meso compounds<br />
Another type of stereoisomer is called a meso compound.<br />
• A meso compound contains at least two stereogenic centers, yet the<br />
molecule itself is not chiral.<br />
• Meso compounds contain an internal plane of symmetry.<br />
H 3 C CH 3<br />
Cl<br />
Cl Br Br<br />
H 3 C CH 3<br />
Cl<br />
Cl<br />
Br Br<br />
.<br />
10
Cahn-Ingold-Prelog Convention <strong>for</strong> Assigning Absolute Configurations<br />
The rules <strong>for</strong> assignment of priorities in order to assign absolute configuration<br />
are based on the same set of rules <strong>for</strong> assigning E <strong>and</strong> Z stereochemistry. Use<br />
the Cahn-Ingold-Prelog scheme to assign priorities to the groups attached to<br />
the carbon atom as follows:<br />
I. Consider each group attached to the carbon in question separately.<br />
II. Rank the priority of the substituent on the carbon as follows.<br />
A. The atom with the higher molecular weight takes top priority. If<br />
there are two isotopes of the same atom, the isotope with the higher<br />
mass takes priority.<br />
B. If this does not distinguish, then move down the chain of the<br />
substituent assigning priorities until you reach the first point of<br />
difference.<br />
III. Multiple bonds count as multiples of that same atom.<br />
IV. Once the priorities have been assigned, rotate the molecule in space so<br />
that the lowest priority group is pointing back.<br />
V. Connect the three remaining groups in order of decreasing priority <strong>and</strong><br />
examine the direction of the resulting rotation.<br />
VI. Clockwise rotation is termed R (rectus; right) <strong>and</strong> counterclockwise<br />
rotation is termed S (sinister; left).<br />
• Alternatively, if the lowest priority is NOT in the back simply switch the<br />
group that is lowest priority with another group. You have just made the<br />
mirror image of the molecule. Now assign R or S to the compound as it<br />
IS drawn, <strong>and</strong> then SWITCH THE R AND S LABEL TO GET THE<br />
ORIGINAL COMPOUNDS.<br />
11
Cahn-Ingold-Prelog Convention <strong>for</strong> Assigning Absolute Configurations<br />
H<br />
Cl<br />
H 3 C<br />
Br<br />
Cl<br />
120° rotation<br />
H 3 C<br />
H<br />
Br<br />
4<br />
2<br />
120° rotation<br />
1<br />
4<br />
3<br />
2<br />
3<br />
1<br />
Counterclockwise = S<br />
12
Fischer Projections<br />
The conversion of a perspective drawing to a Fischer projection requires<br />
rotating the molecule so that the "top" <strong>and</strong> "bottom" groups are oriented back,<br />
away from you as is shown <strong>for</strong> the two molecules below.<br />
H<br />
H<br />
H 3 C<br />
OH<br />
H 3 C<br />
OH<br />
CH 2 OH<br />
CH 2 OH<br />
HO<br />
H<br />
H<br />
HO<br />
H<br />
OH<br />
H<br />
OH<br />
CHO<br />
CH 2 OH<br />
H<br />
HO<br />
H<br />
H<br />
H<br />
OH<br />
OH<br />
CH 2 OH<br />
CHO<br />
OH<br />
A Fischer projection can be used to assign absolute configuration (R or S).<br />
4<br />
4<br />
3<br />
1<br />
3<br />
1<br />
2<br />
4<br />
R<br />
2<br />
4<br />
1<br />
3<br />
1<br />
3<br />
2<br />
S<br />
2<br />
13
A rotation of three groups, as shown below, is equivalent to rotating the<br />
molecule around a single bond (here the CH 3 -C central bond).<br />
H 3 C<br />
H 3 C<br />
HOH 2 C<br />
H<br />
HO<br />
CH 2 OH<br />
OH<br />
H<br />
A 180 degree rotation of the entire molecule regenerates the identical<br />
configuration.<br />
OH<br />
OH<br />
H<br />
CH 2 OH<br />
H<br />
CH 2 OH<br />
CH 3<br />
CH 3<br />
S<br />
CH 3<br />
H 3 C<br />
HOH 2 C<br />
H<br />
HOH 2 C<br />
H<br />
OH<br />
S<br />
OH<br />
Are we having fun yet<br />
14
A 90 degree rotation of the entire molecule generates the enantiomer of the<br />
original molecule.<br />
H<br />
H<br />
H 3 C<br />
OH<br />
H 3 C<br />
OH<br />
CH 2 OH<br />
R<br />
CH 2 OH<br />
OH<br />
OH<br />
H<br />
CH 2 OH<br />
H<br />
CH 2 OH<br />
CH 3<br />
S<br />
CH 3<br />
If you flip the molecule out of the page, you generate the enantiomer of the<br />
original molecule.<br />
H<br />
H<br />
H 3 C<br />
OH<br />
H 3 C<br />
OH<br />
CH 2 OH<br />
R<br />
CH 2 OH<br />
H<br />
H<br />
CH 2 OH<br />
HO CH 3<br />
S<br />
CH 2 OH<br />
HO CH 3<br />
15
Fischer projections are also very convenient <strong>for</strong> identifying whether pairs of<br />
molecules are identical, enantiomers or diastereomers.<br />
For example:<br />
H<br />
H<br />
HO<br />
CH 2 OH<br />
Enantiomers<br />
HOH 2 C<br />
OH<br />
H 3 C<br />
OH<br />
HO<br />
CH 3<br />
H<br />
H<br />
Diastereomers<br />
Diastereomers<br />
Diastereomers<br />
H<br />
H<br />
HO<br />
CH 2 OH<br />
Enantiomers<br />
HOH 2 C<br />
OH<br />
HO<br />
CH 3<br />
H 3 C<br />
OH<br />
H<br />
H<br />
16
In summary:<br />
• Exchanging any two groups around a Fischer projection generates the<br />
enantiomer of the original compound.<br />
• Rotating 90° generates enantiomers.<br />
• Flipping the molecule out of the page generates enantiomers.<br />
• Rotating 180° regenerates the same molecule.<br />
• Rotating three groups is like a rotation about a bond <strong>and</strong> does not change<br />
the configuration.<br />
• Exchanging groups twice regenerates the original stereochemistry.<br />
Review:<br />
• A molecule with one chiral center will be chiral.<br />
• A molecule need not have a chiral center to be chiral.<br />
• A molecule with an inversion center cannot be chiral.<br />
• A molecule with one or more internal mirror planes cannot be chiral.<br />
A molecule can have more than one chiral center <strong>and</strong> not be chiral (if the chiral<br />
centers are symmetry related by a mirror plane or an inversion center).<br />
17
If you have a molecule with two chiral centers with different sets of things<br />
attached (1 <strong>and</strong> 2) either which can be R or S, there are four possible molecules<br />
that can result:<br />
(1R, 2S), (1R, 2R), (1S, 2S), (1S, 2R).<br />
• Since <strong>for</strong> a single chiral center R <strong>and</strong> S are related by mirror symmetry then<br />
if in a molecule all possible centers are switched from R to S <strong>and</strong> vice versa,<br />
then the resultant molecule will be an enantiomer of the original molecule.<br />
• If one or more chiral centers, but not all centers are switched from R to S, the<br />
molecules will not be related by mirror images, <strong>and</strong> there<strong>for</strong>e are not<br />
enantiomers.<br />
Such molecules are called diastereomers <strong>and</strong> they have<br />
different physical <strong>and</strong> chemical properties.<br />
This is illustrated in the example below:<br />
1<br />
2<br />
Cl<br />
Cl<br />
OH Diastereomers OH<br />
(1R, 2R) (1S, 2R)<br />
Enantiomers<br />
Enantiomers<br />
Diastereomers<br />
(1S, 2S) (1R, 2S)<br />
Diastereomers<br />
Cl<br />
OH<br />
Cl<br />
OH<br />
18
If you have a molecule with two chiral centers with the same sets of things<br />
attached (1 <strong>and</strong> 2) either which can be R or S, four possible molecules that can<br />
result:<br />
(1R, 2S), (1R, 2R), (1S, 2S), (1S, 2R)<br />
The following relations will hold as illustrated below:<br />
OH<br />
H 3 C H<br />
H 3 C<br />
1 OH<br />
H<br />
2 H<br />
OH<br />
H 3 C<br />
H 3 C<br />
OH<br />
H<br />
Enantiomers<br />
(1R, 2R) (1S, 2S)<br />
Diastereomers<br />
Diastereomers<br />
(1R, 2S) (1S, 2R)<br />
Identical, MESO<br />
H 3 C<br />
H 3 C<br />
H 3 C<br />
H<br />
OH<br />
OH<br />
H<br />
H 3 C<br />
OH<br />
H<br />
H<br />
OH<br />
The chemical <strong>and</strong> physical properties of diastereomers can be completely<br />
different. Thus, they will react at different rates <strong>and</strong> can have different melting<br />
<strong>and</strong> boiling points etc.<br />
The properties of enantiomers will be identical (so long as they are not<br />
interacting with a chiral perturbation as will be described in more detail later).<br />
Thus, they react with achiral materials at the same rates <strong>and</strong> have identical<br />
melting points.<br />
19
Disubstituted Cyclohexanes<br />
Cis <strong>and</strong> trans isomers can be drawn as planar views that are convenient <strong>for</strong><br />
looking <strong>for</strong> symmetry elements. Thus cis 1,2-dimethylcyclohexane can be<br />
drawn as shown below. Note: that the isomers with both methyl groups up out<br />
of the page is related to that with both into the page by rotating 180° about an<br />
axis as shown below:<br />
Note also that in the planar view there is a mirror plane perpendicular to the<br />
page bisecting the C-C bonds with the methyl groups attached, thus we<br />
conclude that the molecule is achiral. This is not obvious looking at the mirror<br />
image in the chair <strong>for</strong>m until you take into account that the molecule is<br />
con<strong>for</strong>mationally flexible <strong>and</strong> a rotation followed by a chair-chair-flip makes<br />
the mirror images congruent.<br />
20
otate 120°<br />
chair-chair<br />
flip<br />
noncongruent<br />
rotate 120°<br />
Note that if you cooled cis 1,2-diethycyclohexane to a temperature at which the<br />
chair-chair flip was extremely slow, then the two mirror images would be<br />
nonconguent <strong>and</strong> in principle you could separate each of the enantiomers.<br />
In contrast the trans <strong>for</strong>m of 1,2-dimethylcyclohexane has no mirror plane<br />
when drawn in the planar <strong>for</strong>m. It does however have a two-fold axis of rotation<br />
which relates the up <strong>and</strong> down methyl groups.<br />
Since there is no mirror (<strong>and</strong> no inversion center) the trans <strong>for</strong>m is chiral <strong>and</strong><br />
we would there<strong>for</strong>e expect the mirror image not to be superimposable on the<br />
original molecule as shown below.<br />
rotate 120°<br />
rotate 180°<br />
noncongruent<br />
noncongruent<br />
21
1,3-dimethylcyclohexane<br />
• If we consider cis <strong>and</strong> trans 1,3-dimethylcyclohexane then we see again that<br />
the cis isomer has a mirror plane <strong>and</strong> the trans isomer does not.<br />
• Note also that in the chair <strong>for</strong>m both the axial-axial, <strong>and</strong> the equatorialequatorial<br />
<strong>for</strong>ms have mirrors as drawn <strong>and</strong> thus are achiral even when each<br />
<strong>for</strong>m is frozen out at low temperature.<br />
chair-chair<br />
flip<br />
22
Equivalence<br />
Homotopic hydrogens: If you have two identical molecules each with a<br />
methylene group of the <strong>for</strong>m X- CH(1)H(2)-X, <strong>and</strong> you replace one of the<br />
hydrogens of the H(1) in one molecule with a dummy atom (Y) <strong>and</strong> then you<br />
independently replace the H(2) in the other molecule with a dummy atom(Y), if<br />
then the two molecules thus created will be identical to each other, the<br />
hydrogens are said to be homotopic. (e.g. The protons on a methyl group are<br />
homotopic)<br />
H<br />
X<br />
C<br />
X<br />
H<br />
Y<br />
H<br />
X<br />
C<br />
X<br />
X<br />
C<br />
X<br />
H<br />
Y<br />
Identical<br />
Enantiotopic: If you have a methylene group of the <strong>for</strong>m X- CH 2 -Z, <strong>and</strong> you<br />
replace one of the hydrogens of the CH 2 with a dummy atom <strong>and</strong> then you<br />
independently replace the other hydrogen of the CH 2 group with a dummy<br />
atom (Y), the two molecules thus created will be enantiomers of each other.<br />
The protons are said to be enantiotopic. In a nonchiral environment<br />
enantiotopic protons are equivalent. However, in a chiral environment such as<br />
a chiral solvent, they can, in principle, have different chemical shifts.<br />
H<br />
Z<br />
C<br />
X<br />
H<br />
Y<br />
H<br />
Z<br />
C<br />
X<br />
Z<br />
C<br />
X<br />
H<br />
Y<br />
Enantiomers<br />
23
Diastereotopic: If you have a methylene group <strong>for</strong> example, in a chiral<br />
molecule X- CH 2 -Z* (where the * indicates that Z is a chiral group), <strong>and</strong> you<br />
replace one of the hydrogens of the CH 2 with a dummy atom (Y) <strong>and</strong> then you<br />
independently replace the other hydrogen of the CH 2 group with a dummy<br />
atom the two molecules thus created will be diastereomers to each other. Thus,<br />
in principle, the two hydrogens should have different chemical shifts.<br />
H<br />
Z*<br />
C<br />
X<br />
H<br />
Y<br />
H<br />
Z*<br />
C<br />
X<br />
Z*<br />
C<br />
X<br />
H<br />
Y<br />
Diastereomers<br />
24
On The Interaction of Enantiomers with Chiral Perturbations:<br />
A chiral perturbation is any physical or chemical perturbation that has a<br />
h<strong>and</strong>edness.<br />
• Let us assume that we have a pair of chiral acids that are enantiomers. If a<br />
solution is made up of equal amounts of the R <strong>and</strong> S isomers, the mixture is<br />
said to be racemic.<br />
• If sodium hydroxide is added to the solution, then the acids will be<br />
deprotonated <strong>and</strong> will result in a racemic solution of the carboxylate anions.<br />
• We then crystallize them with a chiral cation as shown below:<br />
H 3 C<br />
Enantiomers<br />
H 3 C<br />
H<br />
H 3 C<br />
R<br />
COO<br />
H 3 C<br />
H<br />
S<br />
COO<br />
H 3 C<br />
H 3 C<br />
H<br />
N<br />
S<br />
H 3 C<br />
H 3 C<br />
H 3 C<br />
H 3 C<br />
H<br />
H 3 C<br />
R<br />
COO<br />
H 3 C<br />
H<br />
N<br />
S<br />
H 3 C<br />
H<br />
S<br />
COO<br />
H 3 C<br />
H<br />
N<br />
S<br />
Diastereomers<br />
25
• In such a case the two salts that are <strong>for</strong>med are diastereomers. (Remember:<br />
they will have different physical properties including melting points <strong>and</strong><br />
solubility)<br />
• As a result of these different physical properties, one salt may preferentially<br />
crystallize from the solution leaving the other behind. If the crystallized salt<br />
is isolated <strong>and</strong> then acidified, the chiral acid of just one of the enatiomers<br />
will be regenerated. Such as process is call a chemical resolution of<br />
enantiomers.<br />
Note: There are many examples of interactions of chiral molecules with<br />
chiral perturbations leading to diastereomeric interactions.<br />
• Different molecules can be separated by chromatography. If the stationary<br />
phase is chiral, then each enantiomer in a racemic solution will interact<br />
differently with the stationary phase since the interactions will be<br />
diastereomeric. As a result, each enantiomer may move through the chiral<br />
material at a different speed. Thus, a chiral resolution can be effected.<br />
26
The Interaction of Chiral Molecules with Light<br />
• Plane polarized light is light wherein the electric field oscillates in one plane.<br />
• Plane polarized light can be thought of being made up of a superposition of<br />
two chiral <strong>and</strong> opposite circularly polarized electric fields.<br />
• If we now consider each h<strong>and</strong> of the circularly polarized light interacting<br />
with one enantiomer of the chiral carboxylate that we considered above, we<br />
see that the interaction of the light with the molecule is a diastereomeric<br />
interaction.<br />
• Accordingly, the index of refraction (speed of light in the solution relative to<br />
that of light in a vacuum) <strong>for</strong> the left <strong>and</strong> right circularly polarized light will<br />
be different. Thus, one h<strong>and</strong> of the circularly polarized light will get slowed<br />
down more.<br />
H 3 C<br />
H 3 C<br />
H<br />
H 3 C<br />
R<br />
COO<br />
H<br />
H 3 C<br />
R<br />
COO<br />
diasteriomeric interaction with each<br />
h<strong>and</strong> of circularly polarized light<br />
27
• As a result when the light passes through a cell containing a non-racemic<br />
assembly of chiral molecules, the phase shift of one h<strong>and</strong> of the circularly<br />
polarized light relative to the other will result in the plane of light being<br />
rotated by angle α. Such a solution is optically active. 0<br />
in<br />
α<br />
out<br />
• Since a racemic solution has equal amounts of chiral molecules that have<br />
opposite configurations, <strong>for</strong> each molecule rotating the light in one direction,<br />
there will be a molecule of opposite configuration rotating the light in the<br />
opposite direction. Thus, the two rotations will cancel, <strong>and</strong> there will be no<br />
net rotation of light. Such a solution is not optically active. = 0<br />
Note: A solution of a meso compound is not chiral, <strong>and</strong> it is not racemic. A<br />
racemic solution is made up of an equal mixture of chiral molecules. Since a<br />
meso compound is not chiral, it is not optically active. = 0<br />
28
Amine Inversions<br />
Tertiary amines substituted with three different groups in frozen configurations<br />
are chiral. Thus, their mirror images are not superimposable.<br />
H 3 C<br />
N<br />
H<br />
CH 2OH<br />
HOH 2C<br />
H<br />
N<br />
CH 3<br />
.<br />
However there is a process called amine inversion wherein the substituents on<br />
the nitrogen distort through a plane transition state such that there is an<br />
inversion of configuration:<br />
H 3 C<br />
H<br />
CH 2 OH<br />
H 3 C<br />
N<br />
H<br />
CH 2 OH<br />
H 3 C<br />
N<br />
H<br />
CH 2 OH<br />
29
Such a process creates the enantiomer of the original molecule:<br />
H 3 C<br />
N<br />
H<br />
CH 2 OH<br />
HOH 2 C<br />
H<br />
N<br />
CH 3<br />
H 3 C<br />
N<br />
H<br />
CH 2 OH<br />
• This inversion process, which is an equilibrium, will take a single chiral<br />
isomer into a racemic mixture. Any process that allows one chiral isomer to<br />
interconvert with its enantiomer is termed racemization.<br />
• Since amine inversion can be fast at room temperature, it is often impossible<br />
to isolate one enantiomer of chiral amines. For example in ammonia the<br />
barrier <strong>for</strong> inversion is 5.8 kcal/mol (the rate at ambient temperature is about<br />
2 x 10 11 / sec), <strong>and</strong> <strong>for</strong> methylamine the barrier <strong>for</strong> inversion is 4.8 kcal/mol.<br />
If a chiral amine has the nitrogen tied down into a bicyclic ring system, then it<br />
would be impossible <strong>for</strong> the nitrogen to invert without introducing an<br />
unreasonable amount of strain into the molecule. In such cases, it should be<br />
possible to isolate one enantiomer:<br />
1<br />
N<br />
3<br />
2<br />
R<br />
It is possible to assign configurations to chiral amines. Simply use the Cahn-<br />
Ingold-Prelog convention <strong>and</strong> always assign the lone pair the lowest priority.<br />
30
Stereoselective <strong>and</strong> Stereospecific Reactions.<br />
• Regioselective reaction: A reaction in which one structural isomer is<br />
<strong>for</strong>med preferentially over another. (In some cases, this preference can be<br />
extremely lopsided such that essentially only one isomeric product is<br />
<strong>for</strong>med. In such cases, this is termed a regiospecific reaction.)<br />
• Stereoselective reaction: Is a reaction in which one stereoisomer in a<br />
mixture is created or consumed more quickly than other, such that one<br />
stereoisomeric product is preferentially <strong>for</strong>med.<br />
Note: a reaction can be moderately or very stereoselective.<br />
• Stereospecific reaction: A reaction in which relative chemistry of starting<br />
materials defines, due to the mechanism of the reaction relative<br />
stereochemistry of the products is stereospecific<br />
Note: all stereospecific reactions are stereoselective, but the reverse is not<br />
necessarily so.<br />
Consider the reaction of Br 2 with Z, <strong>and</strong> E-2-butene:<br />
H 3 C<br />
BrH<br />
Br 2<br />
CH 3<br />
H 3 C<br />
CH 3<br />
Br H<br />
meso<br />
H 3 C CH 3 Br 2<br />
HBr<br />
H 3 C<br />
CH 3<br />
Br H<br />
R,R<br />
BrH<br />
CH<br />
H 3 C<br />
3<br />
HBr<br />
S,S<br />
Here the mechansim, i.e. anti addition of the bromine to the double in combination<br />
with the stereochemistry of the starting materials (cis or trans) determines the<br />
stereochemistry of the products.<br />
31
Enantiomeric <strong>and</strong> Diastereomeric Transition States<br />
Achiral molecule reacts with an achiral reactant: If an achiral molecule or<br />
intermediate interacts with an achiral reactant then the transition state will be<br />
either be achiral or enantiomeric (if a chiral center is being <strong>for</strong>med).<br />
Br<br />
H 3 C<br />
Br<br />
H<br />
CH 2 CH 3<br />
H 3 C<br />
Br<br />
H<br />
CH 2 CH 3<br />
R<br />
+<br />
H 3 C CH 2 CH 3<br />
H<br />
Br<br />
S<br />
Enantiomeric transition states have the same energy. Thus, the R <strong>and</strong> S isomer<br />
will <strong>for</strong>m at identical rates <strong>and</strong> a racemic mixture will always result.<br />
R<br />
S<br />
R<br />
Achiral<br />
S<br />
Chiral molecule reacts with a chiral reactant: If a chiral molecule or<br />
intermediate interacts with a chiral reactant then the transition state will be<br />
diastereomeric.<br />
Ph<br />
R<br />
H<br />
CH 3<br />
R<br />
R<br />
Ph<br />
R<br />
H<br />
CH 3<br />
H<br />
B<br />
H<br />
Ph<br />
H<br />
CH 3<br />
B<br />
B<br />
H<br />
CH 3<br />
S<br />
H<br />
PH<br />
CH 3<br />
S H<br />
H<br />
Ph<br />
B<br />
H<br />
CH 3<br />
H<br />
Ph<br />
B<br />
32
Enantiomeric <strong>and</strong> Diastereomeric Transition States (continued)<br />
There<strong>for</strong>e, each transition state will be a different energy <strong>and</strong> the diasteriomeric<br />
products will be <strong>for</strong>med at different rates, <strong>and</strong> they will have different energies.<br />
R RR<br />
S RR<br />
S <strong>and</strong> R<br />
S RR<br />
R RR<br />
Achiral molecule reacts with a chiral resolved reagent: If an achiral<br />
molecule interacts with a chiral resolved reagent (such as an enzyme) in such a<br />
manner that in the transition state one or more chiral centers is being <strong>for</strong>med,<br />
then the transition states <strong>for</strong> the R or S center will be diastereomeric. There<strong>for</strong>e,<br />
the R <strong>and</strong> S product will <strong>for</strong>m at different rates.<br />
fumarase<br />
S<br />
fumarase<br />
S<br />
OH<br />
H 2 O<br />
H<br />
OOC<br />
Achiral<br />
COO<br />
H<br />
H<br />
OOC<br />
HO<br />
H<br />
Achiral<br />
COO<br />
H<br />
OOC<br />
H<br />
S<br />
CH 2 COO<br />
34
Enantiomeric <strong>and</strong> Diastereomeric Transition States (continued)<br />
R RR<br />
S RR<br />
S <strong>and</strong> R<br />
S RR<br />
R RR<br />
In the case of an enzyme this stereoselectivity (i.e. the preferential <strong>for</strong>mation of<br />
one stereoisomer over another) can be very high such that in biological systems<br />
often only one isomer is <strong>for</strong>med.<br />
This means that the two diastereomeric transition states are most likely different<br />
in energy by 3 kcal/mol or more.<br />
Tremendous ef<strong>for</strong>t has been devoted toward developing reagents <strong>and</strong> catalysts<br />
<strong>for</strong> use in organic synthesis that work in much then same manner such that a<br />
chemist can select the chiral configuration of a given center. (A Nobel Prize<br />
was awarded <strong>for</strong> this last year).<br />
35
Inversion of Configuration<br />
In a nucleophilic substitution reaction that is concerted (i.e. bonds are being<br />
made <strong>and</strong> broken at the same time), the nucleophile (Nu) attacks the molecule<br />
from the side opposite from the group that will leave (called the leaving group,<br />
LG) (left).<br />
A<br />
B<br />
A<br />
B<br />
B<br />
A<br />
Nu<br />
LG<br />
Nu<br />
LG<br />
Nu<br />
LG<br />
C<br />
C<br />
C<br />
• As this happens the other groups distort to accommodate the incoming<br />
group <strong>and</strong> the molecule goes through a transition state that is basically<br />
symmetrical (middle).<br />
• Then as the bond <strong>for</strong>ming step with the nucleophile is completed <strong>and</strong> the<br />
leaving group departs, the geometry of the rest of the molecule continues to<br />
relax in such a way to restore tetrahedral geometry about the central carbon<br />
atom (right).<br />
• This process involves a net inversion of configuration as illustrated in the<br />
example below.<br />
Cl<br />
H 3 C<br />
H<br />
Cl<br />
δ<br />
Cl<br />
H CH 3<br />
δ<br />
Cl<br />
Cl<br />
CH 3H<br />
Cl<br />
D<br />
D<br />
D<br />
S<br />
R<br />
• Note that with this degenerate substitution of Cl - <strong>for</strong> Cl - the configuration is<br />
inverted.<br />
36
<strong>Stereochemistry</strong> of "Carbocation" - Addition to Alkenes<br />
C<br />
A<br />
D<br />
E<br />
B<br />
Nu<br />
C<br />
Nu<br />
A<br />
D<br />
B<br />
E<br />
C<br />
A<br />
Nu<br />
E<br />
D<br />
B<br />
C<br />
A<br />
B<br />
D<br />
E Nu<br />
E<br />
D<br />
C<br />
Nu<br />
B<br />
A<br />
E<br />
D<br />
C<br />
B<br />
Nu<br />
A<br />
Nu<br />
C<br />
A<br />
top or bottom<br />
addition<br />
D<br />
E<br />
B<br />
Nu<br />
C<br />
A<br />
Nu<br />
E<br />
D<br />
B<br />
D<br />
E<br />
C<br />
Nu<br />
Nu<br />
A<br />
B<br />
E-Nu E-Nu E-Nu<br />
START<br />
HERE!!<br />
C<br />
A<br />
D<br />
B<br />
C<br />
A<br />
B D<br />
C<br />
D<br />
A<br />
B<br />
E-Nu E-Nu E-Nu<br />
top or bottom<br />
addition<br />
C<br />
A<br />
E<br />
D<br />
B<br />
Nu<br />
A<br />
C<br />
E<br />
B<br />
D<br />
Nu<br />
D<br />
C<br />
E<br />
Nu<br />
Nu<br />
B<br />
A<br />
Nu<br />
C<br />
A<br />
E<br />
D<br />
Nu<br />
B<br />
C<br />
A<br />
Nu<br />
D<br />
E<br />
B<br />
A<br />
C<br />
E Nu<br />
B D<br />
A<br />
C<br />
E<br />
Nu<br />
B<br />
D<br />
C<br />
D<br />
E<br />
B<br />
Nu<br />
A<br />
C<br />
D<br />
E<br />
Nu<br />
B<br />
A<br />
37
<strong>Stereochemistry</strong> of Syn- Addition to Alkenes<br />
A<br />
Y<br />
B<br />
C<br />
Y<br />
X<br />
C<br />
X<br />
Y<br />
C<br />
D<br />
X<br />
A<br />
D<br />
B<br />
D<br />
B<br />
A<br />
rotatate<br />
60°<br />
A<br />
C<br />
B<br />
Y<br />
X<br />
D<br />
A C<br />
X Y<br />
D<br />
B<br />
X<br />
C<br />
D<br />
Y<br />
B<br />
A<br />
X Y X Y X Y<br />
START<br />
HERE!!<br />
A<br />
C<br />
B<br />
D<br />
C<br />
A<br />
B D<br />
C<br />
D<br />
A<br />
B<br />
X Y X Y X Y<br />
A<br />
Y<br />
B<br />
A D B<br />
C<br />
C<br />
D<br />
B<br />
A<br />
C<br />
X<br />
D<br />
Y X<br />
X<br />
Y<br />
rotatate<br />
60°<br />
Y<br />
C<br />
A<br />
X<br />
B<br />
D<br />
C<br />
Y<br />
A<br />
X<br />
D<br />
B<br />
D<br />
X<br />
C<br />
Y<br />
B<br />
A<br />
38
39<br />
B<br />
A<br />
C<br />
D<br />
A<br />
B<br />
D<br />
C<br />
D<br />
B<br />
C<br />
A<br />
B<br />
A<br />
C<br />
D<br />
E<br />
A<br />
B<br />
C<br />
D<br />
E<br />
Nu<br />
E<br />
A<br />
B<br />
C<br />
D<br />
B<br />
Nu<br />
A<br />
E<br />
C<br />
D<br />
D<br />
B<br />
C<br />
A<br />
E<br />
D<br />
B<br />
C<br />
A<br />
E<br />
Nu<br />
B<br />
A<br />
C<br />
D<br />
E<br />
B<br />
C<br />
D<br />
E<br />
Nu<br />
E<br />
A<br />
B<br />
C<br />
D<br />
B<br />
Nu<br />
A<br />
E<br />
C<br />
D<br />
D<br />
B<br />
C<br />
A<br />
E<br />
D<br />
B<br />
C<br />
A<br />
E<br />
Nu<br />
A<br />
E-Nu<br />
Nu<br />
inversion<br />
Nu<br />
E-Nu<br />
E-Nu<br />
Nu<br />
inversion<br />
inversion<br />
Nu<br />
inversion<br />
Nu<br />
Nu<br />
inversion<br />
inversion<br />
E-Nu E-Nu E-Nu<br />
<strong>Stereochemistry</strong> of Anti- Addition to Alkenes<br />
START<br />
HERE
Addition to 1-Methyl Cyclohexane<br />
E Nu<br />
X 2<br />
Hg(OAc) 2<br />
HOX<br />
where X = Br Cl<br />
E<br />
Nu<br />
E<br />
E<br />
Nu<br />
substitution<br />
with<br />
INVERSION<br />
E<br />
Nu<br />
E<br />
Nu<br />
E<br />
E Nu<br />
E<br />
HX (DX)<br />
HX (DX), H 2 O<br />
HX (DX), ROH<br />
where X = Br Cl<br />
Nu<br />
E<br />
E<br />
Nu<br />
syn <strong>and</strong><br />
anti<br />
addition<br />
E<br />
Nu<br />
E<br />
Nu<br />
E<br />
D<br />
D<br />
D<br />
D<br />
Nu<br />
E<br />
E<br />
Nu<br />
E<br />
X<br />
1. X Y<br />
Y 2. step 2<br />
syn addition<br />
E<br />
X<br />
Y, (Y')<br />
X<br />
Y<br />
H 2 BH<br />
OOs(O) 2 O<br />
OMn(O) 2 O<br />
H-H, catalyst<br />
X<br />
Y<br />
X<br />
Y, (Y')<br />
40
E Nu<br />
H<br />
H<br />
X 2<br />
Hg(OAc) 2<br />
HOX<br />
where X = Br Cl<br />
E Nu<br />
H<br />
E<br />
H<br />
E<br />
Nu<br />
substitution<br />
with<br />
INVERSION<br />
E<br />
H<br />
Nu<br />
H E<br />
H<br />
Nu<br />
E<br />
H<br />
E<br />
H<br />
NuH<br />
E<br />
H<br />
H<br />
Nu<br />
H<br />
H<br />
E Nu<br />
HX (DX)<br />
HX (DX), H 2<br />
O<br />
HX (DX), ROH<br />
where X = Br Cl<br />
E Nu<br />
H<br />
E<br />
H<br />
H<br />
E<br />
H<br />
Nu<br />
syn <strong>and</strong><br />
anti<br />
addition<br />
E<br />
H<br />
Nu<br />
E<br />
H<br />
Nu<br />
E<br />
H<br />
D<br />
D<br />
D<br />
D<br />
E<br />
Nu<br />
H<br />
E<br />
Nu<br />
H<br />
E<br />
H<br />
X<br />
Y<br />
H<br />
H<br />
H 2 BH<br />
OOs(O) 2<br />
O<br />
OMn(O) 2 O<br />
H-H, catalyst<br />
H<br />
1. X Y<br />
Y X 2. step 2<br />
H<br />
syn addition<br />
X<br />
H<br />
X<br />
Y<br />
H<br />
Y<br />
Y<br />
X<br />
Addition to Cis Double Bonds<br />
E<br />
H<br />
H<br />
X<br />
Y, (Y')<br />
H<br />
X<br />
H<br />
Y, (Y')<br />
H<br />
Y, (Y')<br />
H<br />
X<br />
H<br />
Y, (Y')<br />
X<br />
H<br />
Homework draw out all the products <strong>for</strong> each of these additions <strong>for</strong> (E)-2-<br />
pentene.<br />
41