Volume 8 Issue 3 (pdf) - Andrew John Publishing Inc
Volume 8 Issue 3 (pdf) - Andrew John Publishing Inc
Volume 8 Issue 3 (pdf) - Andrew John Publishing Inc
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
|<br />
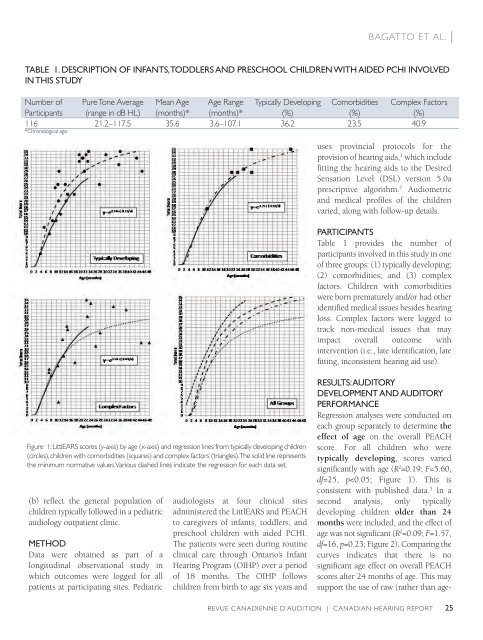
taBLe 1. desCription of infants, toddLers and presChooL ChiLdren With aided pChi invoLved<br />
in this studY<br />
Number of Pure Tone Average Mean Age Age Range Typically Developing Comorbidities Complex Factors<br />
Participants (range in dB Hl) (months)* (months)* (%) (%) (%)<br />
116 21.2–117.5 35.6 3.6–107.1 36.2 23.5 40.9<br />
*Chronological age.<br />
uses provincial protocols for the<br />
provision of hearing aids, 1 which include<br />
fitting the hearing aids to the Desired<br />
Sensation Level (DSL) version 5.0a<br />
prescriptive algorithm. 7 Audiometric<br />
and medical profiles of the children<br />
varied, along with follow-up details.<br />
partiCipants<br />
Table 1 provides the number of<br />
participants involved in this study in one<br />
of three groups: (1) typically developing;<br />
(2) comorbidities; and (3) complex<br />
factors. Children with comorbidities<br />
were born prematurely and/or had other<br />
identified medical issues besides hearing<br />
loss. Complex factors were logged to<br />
track non-medical issues that may<br />
impact overall outcome with<br />
intervention (i.e., late identification, late<br />
fitting, inconsistent hearing aid use).<br />
Figure 1: littlEARS scores (y-axis) by age (x-axis) and regression lines from typically developing children<br />
(circles), children with comorbidities (squares) and complex factors (triangles). The solid line represents<br />
the minimum normative values. Various dashed lines indicate the regression for each data set.<br />
(b) reflect the general population of<br />
children typically followed in a pediatric<br />
audiology outpatient clinic.<br />
Method<br />
Data were obtained as part of a<br />
longitudinal observational study in<br />
which outcomes were logged for all<br />
patients at participating sites. Pediatric<br />
audiologists at four clinical sites<br />
administered the LittlEARS and PEACH<br />
to caregivers of infants, toddlers, and<br />
preschool children with aided PCHI.<br />
The patients were seen during routine<br />
clinical care through Ontario’s Infant<br />
Hearing Program (OIHP) over a period<br />
of 18 months. The OIHP follows<br />
children from birth to age six years and<br />
resuLts: auditorY<br />
deveLopMent and auditorY<br />
perforManCe<br />
Regression analyses were conducted on<br />
each group separately to determine the<br />
effect of age on the overall PEACH<br />
score. For all children who were<br />
typically developing, scores varied<br />
significantly with age (R 2 =0.19; F=5.60,<br />
df=25, p