High performance capillary electrophoresis - T.E.A.M.
High performance capillary electrophoresis - T.E.A.M. High performance capillary electrophoresis - T.E.A.M.
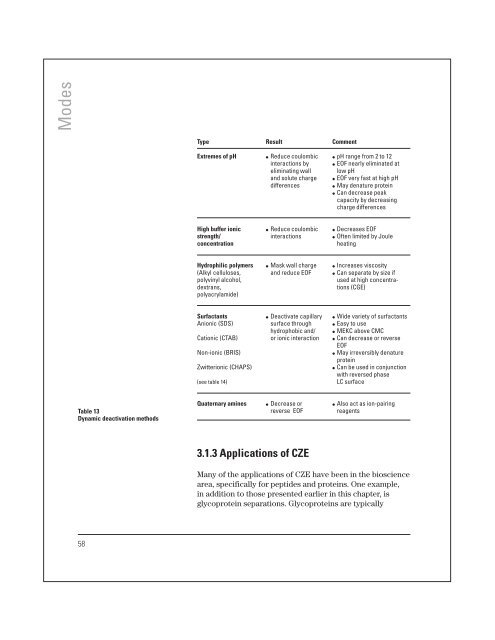
Modes Type Result Comment Extremes of pH ● Reduce coulombic ● pH range from 2 to 12 interactions by ● EOF nearly eliminated at eliminating wall low pH and solute charge ● EOF very fast at high pH differences ● May denature protein ● Can decrease peak capacity by decreasing charge differences High buffer ionic ● Reduce coulombic ● Decreases EOF strength/ interactions ● Often limited by Joule concentration heating Hydrophilic polymers ● Mask wall charge ● Increases viscosity (Alkyl celluloses, and reduce EOF ● Can separate by size if polyvinyl alcohol, used at high concentradextrans, tions (CGE) polyacrylamide) Surfactants ● Deactivate capillary ● Wide variety of surfactants Anionic (SDS) surface through ● Easy to use hydrophobic and/ ● MEKC above CMC Cationic (CTAB) or ionic interaction ● Can decrease or reverse EOF Non-ionic (BRIS) ● May irreversibly denature protein Zwitterionic (CHAPS) ● Can be used in conjunction with reversed phase (see table 14) LC surface Table 13 Dynamic deactivation methods Quaternary amines ● Decrease or ● Also act as ion-pairing reverse EOF reagents 3.1.3 Applications of CZE Many of the applications of CZE have been in the bioscience area, specifically for peptides and proteins. One example, in addition to those presented earlier in this chapter, is glycoprotein separations. Glycoproteins are typically 58
Modes a) CH 3COONa-CH 3COOH difficult to analyze by the traditional techniques of slab gel electrophoresis, isoelectric focusing, or liquid chromatography. In some cases CZE is advantageous. Shown in figure 27 is the separation of glycoforms of the human recombinant protein hormone, erythropoietin. The different species result from heterogeneity after post-translational modification. CZE is well suited for such analyses since many post translational modifications have an impact on protein charge (that is, N- or C- terminal modifications, phosphorylation, carboxylation, or N-glycosylation). b) CH 3COONa-H 3PO c) CH 3COONa-H 2SO4 0 5 10 15 Time [min] Figure 27 Influence of buffer composition on separation of erythropoietin glycoforms 13 Conditions: Buffer concentration = 100 mM, pH 4, V = 10 kV, i = 10, 120, 200 mA in a, b, and c, respectively, l = 20 cm, L = 27 cm, id = 75 mm, l = 214 nm Significant success has been realized for peptide mapping by CZE. In peptide mapping a protein is enzymatically or chemically cleaved into smaller peptide fragments and subsequently separated. The analysis is primarily qualitative and is used to detect subtle differences in proteins. A typical CZE peptide map is shown in figure 28. CZE is also useful as a second-dimension analysis of HPLC-purified peptides (figure 29). mAU 30 28 n 20 Reproducibility (RSD %) Migration time 2.5% Mobility 0.3% 26 24 Figure 28 Rapid BSA peptide map Conditions: 20 mM phosphate, pH 7, V = 25 kV, i = 16 mA, l = 50 cm, L = 57 cm, id = 50 mm with 3X extended pathlength detection cell, l = 200 nm 22 20 18 5 6 7 8 9 10 11 12 Time [min] 59
- Page 8 and 9: Table of content Foreword .........
- Page 10 and 11: Scope The purpose of this book is t
- Page 12 and 13: Introduction 1.1 High performance c
- Page 14 and 15: Introduction sis, methods for on-ca
- Page 16 and 17: Principles 2.1 Historical backgroun
- Page 18 and 19: Principles that ion. The mobility i
- Page 20 and 21: Principles the exact pI of fused si
- Page 22 and 23: Principles µ EOF × 10 -4 (cm 2 /
- Page 24 and 25: Principles For the analysis of smal
- Page 26 and 27: Principles µ EOF ( × 10 -4 cm 2 /
- Page 28 and 29: Principles Total length Effective l
- Page 30 and 31: Principles Note that equation (15)
- Page 32 and 33: Principles determined by the capill
- Page 34 and 35: Principles Current (uA) 300 250 200
- Page 36 and 37: Principles The contribution of inje
- Page 38 and 39: Principles k' H N H, µm 0.001 0.58
- Page 40 and 41: Principles Figure 19 Electrodispers
- Page 42 and 43: Principles rapidly eluting ions, th
- Page 44 and 45: Principles 44
- Page 46 and 47: Modes Mode Capillary zone electroph
- Page 48 and 49: Modes 3.1.1 Selectivity and the use
- Page 50 and 51: Modes Name pK a Phosphate 2.12 (pK
- Page 52 and 53: Modes EOF No flow Figure 22 Elimina
- Page 54 and 55: Modes Absorbance 214 nm 0.05 0.04 0
- Page 56 and 57: Modes Type Comment Silylation coupl
- Page 60 and 61: Modes Figure 29 CZE of reversed pha
- Page 62 and 63: Modes Figure 33 Ion analysis of fer
- Page 64 and 65: Modes The separation mechanism of n
- Page 66 and 67: Modes the stationary phase in LC. S
- Page 68 and 69: Modes Amplitude 2 a) with a migrati
- Page 70 and 71: Modes CGE t = 0 t > 0 Polymer matri
- Page 72 and 73: Modes Crosslinked polyacrylamide, a
- Page 74 and 75: Modes a) ds 500 base pairs This sam
- Page 76 and 77: Modes and resolution with respect t
- Page 78 and 79: Modes 3.5 Capillary isotachophoresi
- Page 80 and 81: Modes 80
- Page 82 and 83: Instrumentation/Operation Diode-arr
- Page 84 and 85: Instrumentation/Operation Pressure
- Page 86 and 87: Instrumentation/Operation If sensit
- Page 88 and 89: Instrumentation/Operation Despite q
- Page 90 and 91: Instrumentation/Operation T, ˚C 10
- Page 92 and 93: Instrumentation/Operation 4.2.1.1 C
- Page 94 and 95: Instrumentation/Operation However,
- Page 96 and 97: Instrumentation/Operation Calculati
- Page 98 and 99: Instrumentation/Operation Method Ma
- Page 100 and 101: Instrumentation/Operation 4.3.3 Lin
- Page 102 and 103: Instrumentation/Operation Area (arb
- Page 104 and 105: Instrumentation/Operation 4.3.6 Ext
- Page 106 and 107: Instrumentation/Operation light int
Modes<br />
Type Result Comment<br />
Extremes of pH ● Reduce coulombic ● pH range from 2 to 12<br />
interactions by<br />
● EOF nearly eliminated at<br />
eliminating wall low pH<br />
and solute charge ● EOF very fast at high pH<br />
differences<br />
● May denature protein<br />
● Can decrease peak<br />
capacity by decreasing<br />
charge differences<br />
<strong>High</strong> buffer ionic ● Reduce coulombic ● Decreases EOF<br />
strength/ interactions ● Often limited by Joule<br />
concentration<br />
heating<br />
Hydrophilic polymers ● Mask wall charge ● Increases viscosity<br />
(Alkyl celluloses, and reduce EOF ● Can separate by size if<br />
polyvinyl alcohol,<br />
used at high concentradextrans,<br />
tions (CGE)<br />
polyacrylamide)<br />
Surfactants ● Deactivate <strong>capillary</strong> ● Wide variety of surfactants<br />
Anionic (SDS) surface through ● Easy to use<br />
hydrophobic and/<br />
● MEKC above CMC<br />
Cationic (CTAB) or ionic interaction ● Can decrease or reverse<br />
EOF<br />
Non-ionic (BRIS)<br />
● May irreversibly denature<br />
protein<br />
Zwitterionic (CHAPS)<br />
● Can be used in conjunction<br />
with reversed phase<br />
(see table 14)<br />
LC surface<br />
Table 13<br />
Dynamic deactivation methods<br />
Quaternary amines ● Decrease or ● Also act as ion-pairing<br />
reverse EOF<br />
reagents<br />
3.1.3 Applications of CZE<br />
Many of the applications of CZE have been in the bioscience<br />
area, specifically for peptides and proteins. One example,<br />
in addition to those presented earlier in this chapter, is<br />
glycoprotein separations. Glycoproteins are typically<br />
58