4ì 20ì¼
4ì 20ì¼
4ì 20ì¼
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
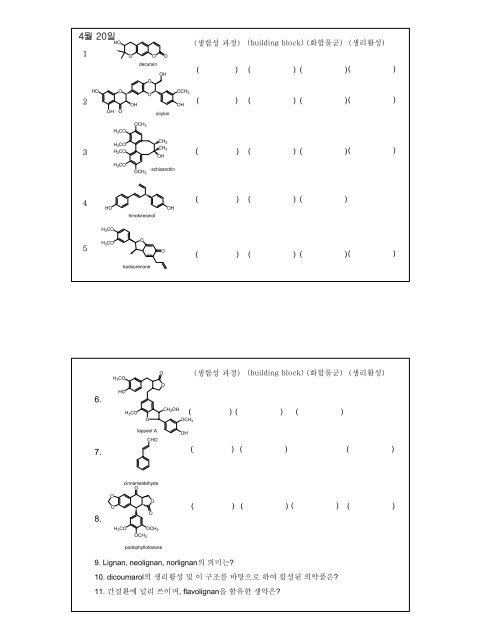
4월 20일<br />
1<br />
HO<br />
O<br />
O<br />
O<br />
(생합성 과정)<br />
(building block) (화합물군)<br />
(생리활성)<br />
decursin<br />
OH<br />
O<br />
( ) ( )<br />
( )( )<br />
2<br />
HO<br />
OH<br />
O<br />
O<br />
OH<br />
O<br />
silybin<br />
OCH 3<br />
OH<br />
( ) ( )<br />
( )( )<br />
H 3 CO<br />
OCH 3<br />
3<br />
H 3 CO<br />
H 3 CO<br />
CH 3<br />
CH 3<br />
OH<br />
( ) ( )<br />
( )( )<br />
H 3 CO<br />
OCH 3<br />
schizandrin<br />
4<br />
HO<br />
hinokiresinol<br />
OH<br />
( ) ( )<br />
( )<br />
H 3 CO<br />
5<br />
H 3 CO<br />
O<br />
O<br />
( ) ( )<br />
( )( )<br />
kadsurenone<br />
6.<br />
H 3 CO<br />
HO<br />
H 3 CO<br />
O<br />
O<br />
O<br />
CH 2 OH<br />
(생합성 과정) (building block) (화합물군)<br />
( ) ( ) ( )<br />
OCH 3<br />
(생리활성)<br />
7.<br />
lappaol A<br />
CHO<br />
OH<br />
( ) ( ) ( )<br />
8.<br />
cinnamaldehyde<br />
O<br />
O<br />
O<br />
O<br />
O<br />
H 3 CO OCH 3<br />
OCH 3<br />
( ) ( ) ( ) ( )<br />
podophyllotoxone<br />
9. Lignan, neolignan, norlignan의 의미는<br />
10. dicoumarol의 생리활성 및 이 구조를 바탕으로 하여 합성된 의약품은<br />
11. 간질환에 널리 쓰이며, flavolignan을 함유한 생약은
Mevalonate pathway<br />
CO 2<br />
H 2 O<br />
hv<br />
photosynthesis<br />
mono-, oligo-,<br />
polyosides<br />
1. Monoterpenoids<br />
erythrose-4<br />
phosphate<br />
glucose<br />
GLYCOSIDES<br />
2. Iridoids and Secoiridoids<br />
shikimate<br />
flavonoids<br />
anthocyanins<br />
tannins…<br />
phospho-enol<br />
pyruvate<br />
phenols, quinones,<br />
polyacetylenes<br />
macrolides, fatty acids<br />
lipids…<br />
POLYACETATES<br />
3. Sesquiterpenoids<br />
4. Diterpenoids<br />
SHIKIMATES<br />
pyruvate<br />
acetyl CoA<br />
mevalonate<br />
5. Sesterterpenoids<br />
cinnamates,<br />
lignans,<br />
coumarins<br />
quinones<br />
cycle<br />
amino acid<br />
TERPENES<br />
AND<br />
STEROLS<br />
6. Triterpenoids and Steroids<br />
proteins…<br />
ALKALOIDS<br />
essential oils,<br />
sesqui- and diterpenes,<br />
saponins, cardenolides<br />
carotenes…<br />
Mevalonate pathway<br />
HOOC<br />
OH<br />
mevalonic acid<br />
OH<br />
dimethylallyl PP<br />
(DMAPP) (C5)<br />
OPP<br />
OPP<br />
isopentenyl PP<br />
(IPP) (C5)<br />
Hemiterpenes (C5)<br />
IPP<br />
geranyl PP<br />
(GPP) (C10)<br />
OPP<br />
Monoterpenes (C10)<br />
IPP<br />
IPP<br />
OPP<br />
farnesyl PP<br />
(FPP) (C15)<br />
geranylgeranyl PP<br />
OPP<br />
(GGPP) (C20)<br />
Sesquiterpenes (C15)<br />
Diterpenes (C20)<br />
geranylfarnesyl PP<br />
OPP<br />
(GFPP) (C25)<br />
Sesterpenes (C25)<br />
2 x FPP squalene<br />
Triterpenoids (C30)<br />
Steroids (C18-C30)<br />
2 x GGPP phytoene<br />
Tetraterpenes (C40)<br />
carotenoids
Mevalonate pathway<br />
O<br />
HOOC<br />
SCoA<br />
H<br />
SCoA<br />
O<br />
OH<br />
OH<br />
mevalonic acid<br />
(MVA)<br />
Claisen<br />
reaction<br />
EnzSH<br />
acetyl-CoA<br />
O<br />
reduction of aldehyde<br />
to alcohol<br />
NADPH<br />
HOOC<br />
O<br />
SCoA<br />
SEnz<br />
O<br />
Enzyme-bound<br />
acetyl group<br />
OH OH<br />
mevaldic acid<br />
stereospecific aldol reaction;<br />
also involves hydrolysis of<br />
acetyl-enzyme linkage<br />
O<br />
H<br />
HOOC<br />
OH O<br />
+ EnzSH<br />
SCoA<br />
HMG-CoA<br />
HMG-CoA<br />
reductase NADPH<br />
reduction of thioester to<br />
aldehyde via hemiacetal<br />
OH OH<br />
HOOC<br />
SCoA<br />
H<br />
mevaldic acid<br />
hemithioacetal<br />
sequential<br />
phosphorylation ot the<br />
primary alcohol to a<br />
diphosphate<br />
O<br />
H<br />
O<br />
OH<br />
2 x ATP<br />
O<br />
HO P O ADP<br />
OH<br />
OP<br />
ATP<br />
-CO 2<br />
H<br />
4<br />
5<br />
stereospecific allylic<br />
isomerization; equilibrium<br />
favors DMAPP<br />
3<br />
1<br />
2<br />
OPP<br />
H R H S<br />
isomerase<br />
OPP<br />
ATP facilitates the<br />
decarboxylation-eliminatio<br />
n; the anticipated<br />
phosphorylation of the<br />
tertiary alcohol to make a<br />
better leaving group is<br />
apparently not involved<br />
isopentenyl PP<br />
(IPP)<br />
dimethylallyl PP<br />
(DMAPP)<br />
Acetate-malonate pathway (= Polyketide pathway)<br />
1.General reactions in acetogenins<br />
O O<br />
H 3 C C CH 2 C SCoA<br />
B A<br />
acetoacetyl-CoA<br />
A<br />
+<br />
O<br />
H 3 C C SCoA<br />
acetyl-CoA<br />
B<br />
HO CH 2 COOH<br />
C<br />
H 3 C CH 2 C SCoA<br />
O<br />
O O O<br />
H 3 C C CH 2 C CH 2 C SCoA<br />
isoprenoid<br />
cyclization<br />
reduction<br />
aromatics<br />
fatty acids
Each group of terpenes arises from the head-to-tail condensation of a<br />
variable number of isopren unit<br />
electrophile addition<br />
giving tertiary cation<br />
OPP<br />
dimethylallyl PP<br />
(DMAPP)<br />
OPP<br />
H R H S<br />
isopentenyl PP<br />
(IPP)<br />
OPP<br />
H R H S<br />
stereospecific<br />
loss of proton<br />
OPP<br />
OPP<br />
geranyl PP<br />
(GPP)<br />
Origin of terpenes: formation of the precusors of each class<br />
IPP or DMAPP<br />
DMAPP + IPP = GPP<br />
GPP + IPP= FPP<br />
FPP+ IPP= GGPP<br />
GGPP + IPP= GFPP<br />
2 x FPP = Squalene<br />
2x GGPP = cis phytoene<br />
hemiterpene (C 5<br />
)<br />
monoterpene (C 10<br />
)<br />
sesquiterpene (C 15<br />
)<br />
diterpenes (C 20<br />
)<br />
sesterpenes (C 25<br />
)<br />
triterpene (C 30<br />
)<br />
tetraterpene (C 40<br />
) --- carotenoid<br />
1. Monoterpenes<br />
GPP (generanyl pyrophosphate)가 전구체(C10)<br />
주로 식물에서 발견- 동물이나 미생물에서도 발견<br />
식물 정유의 구성성분으로 존재하기 때문에 수증기 증류나 용매 추출에 의해 분리<br />
구조<br />
- acyclic (myrcane or 2,6-dimethyloctane, secoiridane)<br />
- monocyclic (e.g., p-methane, iridane)<br />
- bi- and trichclic (e.g., carane, pinane, bornane, thuyane)<br />
myrcane p-menthane seco-iridane iridane carane<br />
pinene thujane bornane fenchane iso-camphane<br />
artemisane santolinane chrysanthemane lavandulane<br />
main structureal classes of monoterpenoids
camphor: 녹나무 (Cinnamomun camphor)에서 얻어진 것, 국소자극으로 신경통, 염증,타박상<br />
등에대한찰제, 중추자극작용<br />
menthol: 박하 (Mentha arvensis) 또는 양박하(Mentha piperita)의 정유중에 함유, 피부에 냉<br />
감작용, 약한 마취작용, 진통, 제양작용<br />
paeoniflorin: 적작약 (Paeonia lactiflora)에서 얻어낸 monoterpene glycoside의 benzoate,<br />
진통, 진경, 항염증<br />
limonene: lemon의 정유성분, 진정, 중추억제 작용<br />
fenchon: 회향 (Foeniculum vulgare), 딱정벌레류에 대해서 살충작용<br />
1) acyclic monoterpene 2) monocyclic monoterpene 3) bicyclic monoterpene<br />
OH<br />
OH<br />
OH<br />
OH<br />
OH<br />
O<br />
OH<br />
O<br />
citronellol<br />
(rose oil)<br />
geraniol<br />
(geranium oil)<br />
nerol<br />
(rose oil)<br />
limonene<br />
α-terpineol<br />
(-)-menthol<br />
(levomenthol)<br />
thujone<br />
fenchol<br />
fenchone<br />
β-myrcene<br />
(hops)<br />
O<br />
neral (E-citral)<br />
(lemon oil)<br />
O<br />
geranial (Z-citral)<br />
(lemon oil)<br />
thymol<br />
OH<br />
HO<br />
charvacrol<br />
p-cymene<br />
cineol<br />
O<br />
O<br />
camphor<br />
α-pinene<br />
Irregular monoterpene<br />
GPP를 전구체로 하지 않고 2분자의 DMAPP가 축합하여 생성되는 monoterpenes<br />
-pyrethrins: 제충국 (Chrysanthemum cineraiefolium)등의 꽃에 함유<br />
- pyrethrins, cinerin, jasmolin<br />
- 유용한 살충성분<br />
OPP<br />
electrophilic addition<br />
giving tertiary cation<br />
H<br />
OPP<br />
OPP<br />
loss of proton via<br />
cyclopropyl ring<br />
formation<br />
hydrolysis of phosphate ester;<br />
oxidation of alcohol to acid<br />
OPP<br />
COOH<br />
crysanthemic acid<br />
cf. regular monoterpene skeleton<br />
R 1<br />
O<br />
O<br />
R 2<br />
O<br />
pyrenthrin I, R 1 =CH 3 , R 2 =CH=CH 2<br />
pyrenthrin II, R 1 =COOCH 3 , R 2 =CH=CH 2<br />
cinerin I, R 1 =CH 3 , R 2 =CH 3<br />
cinerin II, R 1 =COOCH 3 , R 2 =CH 3<br />
jasmolin I, R 1 =CH 3 , R 3 =CH 2 CH 3<br />
jasmolin II, R 1 =COOCH 3 , R 2 =CH 2 CH 3<br />
HO<br />
COOH<br />
crysanthemic acid<br />
HO<br />
O<br />
pyrethrolone<br />
COOH<br />
H 3 COOC<br />
pyrethric acid<br />
HO<br />
O<br />
O<br />
cinerolone<br />
jasmolone<br />
O<br />
O N<br />
O<br />
teramethrin<br />
O<br />
O<br />
O<br />
O<br />
bioresmethrin<br />
Cl<br />
Cl<br />
O<br />
O<br />
R<br />
O<br />
permethrin, R=H<br />
cypermethrin, R=CN
2. Iridoids and Secoiridoids<br />
iridoid: cyclopentane ring이 있음<br />
<br />
<br />
GPP로부터 생합성 됨<br />
쌍자엽 식물에 주로 분포됨<br />
6<br />
7<br />
5<br />
8 9<br />
10<br />
11<br />
4<br />
3<br />
O<br />
1<br />
기본핵 iridoid secoiridoid<br />
geraniol<br />
via hydroxylation<br />
and oxidation<br />
OH<br />
H<br />
O<br />
CHO<br />
H<br />
CHO<br />
H<br />
CHO<br />
iridodial<br />
(keto form)<br />
cyclization formulated as initiated by electrophilic<br />
addition utilizing the unsaturated carbonyl, terminated by<br />
addition of hydride; the Schiff base-assisted mechanism<br />
shown below is more realistic<br />
hemiacetal<br />
formation<br />
H<br />
H<br />
O<br />
OH<br />
iridodial<br />
(enol form)<br />
H<br />
OH<br />
=<br />
H<br />
O<br />
iridodial<br />
(hemiacetal form)<br />
H<br />
O<br />
H<br />
OH<br />
harpagoside- 현삼, 소염진통작용<br />
catalpol- 지황, 혈압강하작용<br />
linaroside- 운란,완화( 緩 和 )작용<br />
geniposide- 치자, 완화, 이담, 지혈, 소염, 강장<br />
해독, 해열<br />
villoside: 패장<br />
loganin, gentioside: 수채엽<br />
syringopicroside: 야정향<br />
valtrate, dehydrovaltrate: 길초근, 진정, 불면증<br />
완화<br />
muzenide: 여정실<br />
picroside I, II, III: 호황련<br />
HO<br />
RO<br />
OH<br />
O<br />
H<br />
OGlc<br />
harpagoside, R=t-cinnamoyl<br />
HO<br />
COOCH 3<br />
H<br />
O<br />
H OGlc<br />
loganin<br />
COOCH<br />
H<br />
3<br />
O<br />
HOH H 2 C OR<br />
genipin, R=H<br />
geniposide, R=Glc<br />
HO<br />
Cl<br />
HO<br />
OH<br />
O<br />
H OGlc<br />
linarioside<br />
CH 2 O-Glc RO<br />
H<br />
H<br />
O<br />
O<br />
O<br />
H HOH<br />
H<br />
O 2 C OGlc<br />
catalpol, R=H<br />
villoside<br />
catalposide, R=p-hydroxybenzoyl<br />
OAc<br />
O<br />
O<br />
O<br />
O<br />
O<br />
H<br />
O<br />
AcO<br />
O<br />
O<br />
H<br />
O<br />
O<br />
HO<br />
O O<br />
H<br />
O<br />
H OGlc<br />
OH<br />
HO<br />
HO<br />
H<br />
O<br />
H OGlc<br />
HO<br />
HO<br />
COOCH 3<br />
H<br />
O<br />
H OGlc<br />
valtrate<br />
O<br />
O<br />
dihydrovaltrate<br />
syringopicroside<br />
aucubin<br />
gardenoside
Secoiridoid:<br />
iridoid의 7,8번 탄소사이가 개환된 상태<br />
Gentianaceae, Rubiaceae, Oleaceae, Cornaceae에 많이존재<br />
secologanin: 인동과 식물 등에 존재, 모든 secoiridoid의 중간체<br />
monoterpene indole alkaloid의 생합성에 중요한 전구체임<br />
용담, 겐티아나, 당약- gentiopicroside, swertmarin, sweroside, amorogentin<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
O<br />
OGlc<br />
R<br />
O<br />
H<br />
OGlc<br />
OH<br />
O<br />
OH OH<br />
O<br />
O<br />
O<br />
O<br />
OH<br />
O<br />
ORO<br />
O<br />
O<br />
OAc<br />
O<br />
CH 2 OAc<br />
OH<br />
OGlc<br />
gentiopicroside<br />
swertiamarin, R=OH<br />
sweroside, R=H<br />
HO OH<br />
amarogentin<br />
trifloroside R=COCH 3<br />
benzoyltrifloriside R= CO<br />
4월 22일<br />
OAc<br />
(생합성 과정)<br />
(building block) (화합물군)<br />
(생리활성)<br />
O<br />
1.<br />
O<br />
O<br />
valtrate<br />
O<br />
H<br />
O<br />
O<br />
( ) ( )<br />
( )( )<br />
O<br />
O<br />
2.<br />
O<br />
gentiopicroside<br />
( ) ( )<br />
( )( )<br />
OGlc<br />
3.<br />
COOH<br />
crysanthemic acid<br />
( ) ( ) ( )<br />
4.<br />
OH<br />
(-)-menthol<br />
(levomenthol)<br />
( ) ( ) ( )
5.<br />
Origin of terpenes: formation of the precusors of each class<br />
IPP or DMAPP<br />
DMAPP + IPP = GPP<br />
GPP + IPP= FPP<br />
FPP+ IPP= GGPP<br />
GGPP + IPP= GFPP<br />
2 x FPP = Squalene<br />
2x GGPP = cis phytoene<br />
hemiterpene (C 5<br />
)<br />
monoterpene (C 10<br />
)<br />
sesquiterpene (C 15<br />
)<br />
diterpenes (C 20<br />
)<br />
sesterpenes (C 25<br />
)<br />
triterpene (C 30<br />
)<br />
tetraterpene (C 40<br />
) --- carotenoid