Biophysical studies of membrane proteins/peptides. Interaction with ...
Biophysical studies of membrane proteins/peptides. Interaction with ...
Biophysical studies of membrane proteins/peptides. Interaction with ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
INTRODUCTION: BIOMEMBRANES<br />
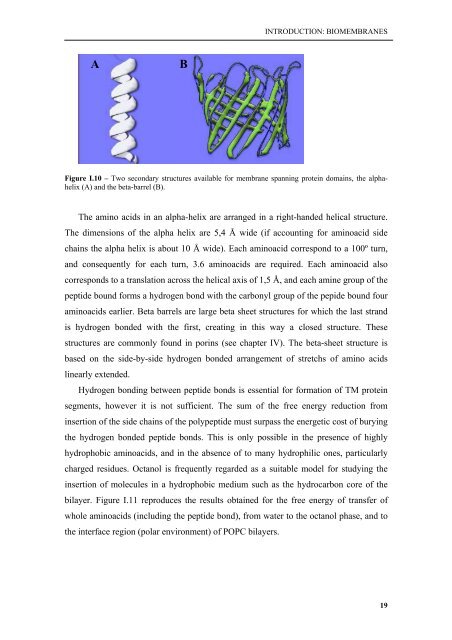
A<br />
B<br />
Figure I.10 – Two secondary structures available for <strong>membrane</strong> spanning protein domains, the alphahelix<br />
(A) and the beta-barrel (B).<br />
The amino acids in an alpha-helix are arranged in a right-handed helical structure.<br />
The dimensions <strong>of</strong> the alpha helix are 5,4 Å wide (if accounting for aminoacid side<br />
chains the alpha helix is about 10 Å wide). Each aminoacid correspond to a 100º turn,<br />
and consequently for each turn, 3.6 aminoacids are required. Each aminoacid also<br />
corresponds to a translation across the helical axis <strong>of</strong> 1,5 Å, and each amine group <strong>of</strong> the<br />
peptide bound forms a hydrogen bond <strong>with</strong> the carbonyl group <strong>of</strong> the pepide bound four<br />
aminoacids earlier. Beta barrels are large beta sheet structures for which the last strand<br />
is hydrogen bonded <strong>with</strong> the first, creating in this way a closed structure. These<br />
structures are commonly found in porins (see chapter IV). The beta-sheet structure is<br />
based on the side-by-side hydrogen bonded arrangement <strong>of</strong> stretchs <strong>of</strong> amino acids<br />
linearly extended.<br />
Hydrogen bonding between peptide bonds is essential for formation <strong>of</strong> TM protein<br />
segments, however it is not sufficient. The sum <strong>of</strong> the free energy reduction from<br />
insertion <strong>of</strong> the side chains <strong>of</strong> the polypeptide must surpass the energetic cost <strong>of</strong> burying<br />
the hydrogen bonded peptide bonds. This is only possible in the presence <strong>of</strong> highly<br />
hydrophobic aminoacids, and in the absence <strong>of</strong> to many hydrophilic ones, particularly<br />
charged residues. Octanol is frequently regarded as a suitable model for studying the<br />
insertion <strong>of</strong> molecules in a hydrophobic medium such as the hydrocarbon core <strong>of</strong> the<br />
bilayer. Figure I.11 reproduces the results obtained for the free energy <strong>of</strong> transfer <strong>of</strong><br />
whole aminoacids (including the peptide bond), from water to the octanol phase, and to<br />
the interface region (polar environment) <strong>of</strong> POPC bilayers.<br />
19