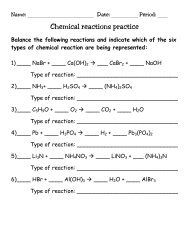
Naming (Ionic) Compounds Practice Sheet
Naming (Ionic) Compounds Practice Sheet
Naming (Ionic) Compounds Practice Sheet
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Name: ______________________________ Date: ____________ Block: _____<br />
<strong>Naming</strong> (<strong>Ionic</strong>) <strong>Compounds</strong> <strong>Practice</strong> <strong>Sheet</strong><br />
Give the name of the following ionic compounds:<br />
Name<br />
1) Na 2 CO 3 ____________________________________________________<br />
2) NaOH _____________________________________________________<br />
3) MgBr 2 _____________________________________________________<br />
4) KCl _______________________________________________________<br />
5) FeCl 2 ______________________________________________________<br />
6) FeCl 3 ______________________________________________________<br />
7) Zn(OH) 2 ___________________________________________________<br />
8) Be 2 SO 4 ___________________________________________________<br />
9) CrF 2 ______________________________________________________<br />
10) Al 2 S 3 _____________________________________________________<br />
11) PbO ______________________________________________________<br />
12) Li 3 PO 4 ____________________________________________________<br />
13) TiI 4 _______________________________________________________<br />
14) Co 3 N 2 ____________________________________________________<br />
15) Mg 3 P 2 ____________________________________________________<br />
16) Ga(NO 2 ) 3 __________________________________________________<br />
17) Ag 2 SO 3 ____________________________________________________<br />
18) NH 4 OH ____________________________________________________<br />
19) Al(CN) 3 ____________________________________________________
<strong>Naming</strong> (Covalent) <strong>Compounds</strong> <strong>Practice</strong> <strong>Sheet</strong><br />
Write the formulas for the following covalent compounds:<br />
1) antimony tribromide __________________________________<br />
2) hexaboron silicide __________________________________<br />
3) chlorine dioxide __________________________________<br />
4) hydrogen iodide __________________________________<br />
5) iodine pentafluoride __________________________________<br />
6) dinitrogen trioxide __________________________________<br />
7) ammonia __________________________________<br />
8) phosphorus triiodide __________________________________<br />
Write the names for the following covalent compounds:<br />
9) P 4 S 5 __________________________________<br />
10) O 2 __________________________________<br />
11) SeF 6 __________________________________<br />
12) Si 2 Br 6 __________________________________<br />
13) SCl 4 __________________________________<br />
14) CH 4 __________________________________<br />
15) B 2 Si __________________________________<br />
16) NF 3 __________________________________