MQP â 014 EQA HANDBOOK EDITION 6 A participant ... - Thistle QA
MQP â 014 EQA HANDBOOK EDITION 6 A participant ... - Thistle QA
MQP â 014 EQA HANDBOOK EDITION 6 A participant ... - Thistle QA
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Clinical Microbiology<br />
Pregnancy<br />
ESR<br />
April & October<br />
P.O. Box 131375, Bryanston, 2074<br />
Ground Floor, Block 5<br />
Bryanston Gate, 170 Curzon Road<br />
Bryanston, Johannesburg, South Africa<br />
804 Flatrock, Buiten Street, Cape Town, 8001<br />
www.thistle.co.za<br />
Tel: +27 (011) 463 3260<br />
Fax: +27 (011) 463 3036<br />
Fax to Email: + 27 (0) 86-557-2232<br />
e-‐mail : service@thistle.co.za<br />
<strong>MQP</strong>-‐<strong>014</strong><br />
Edition 10<br />
Food Micro<br />
May & November<br />
Infectious Disease<br />
June & December<br />
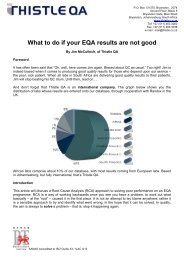
How do the <strong>E<strong>QA</strong></strong>s operate<br />
Provided you have enrolled timeously, your kit will be dispatched approximately<br />
2 weeks before the start date of the new cycle. Bear in mind that samples are<br />
imported and there may at times be customs issues which we do not have<br />
control over, but we will however endeavour at all times to get the samples out<br />
to you on time. Your kit usually has enough samples to cover a six month period.<br />
With the kit comes an instruction sheet, method questionnaire and results entry<br />
sheet for you to fill in.<br />
This results entry sheet already has your own unique <strong>QA</strong> Number on it.<br />
Please choose the method of your choice for each analyte listed on the method<br />
questionnaire and return together with your results to enable correct<br />
registration.<br />
In addition, we send you a Participation Certificate.<br />
The instruction sheet has lots of important information and it must be read<br />
carefully. For example,<br />
Ø Dates of analysis, or the final date by which we must receive your results.<br />
Ø Safety and disposal details for handling the sample<br />
Ø Reconstitution or mixing details<br />
Ø The important recommendation that you analyse our samples exactly as if<br />
they were patient samples<br />
Ø Storage details<br />
Ø Factors that could affect the testing if applicable<br />
Ø Environmental conditions<br />
Ø Recording and reporting of results<br />
Ø Contact details for any enquiries<br />
Ø Return policy of samples when applicable<br />
Page 5 of 12