Recent advances in plant hepatoprotectives - CIMAP Staff - Central ...
Recent advances in plant hepatoprotectives - CIMAP Staff - Central ...
Recent advances in plant hepatoprotectives - CIMAP Staff - Central ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
RECENT ADVANCES IN PLANT HEPATOPROTECTIVES<br />
*<br />
751<br />
O<br />
O<br />
15<br />
O<br />
14 16<br />
HO 13<br />
O<br />
11 12<br />
1<br />
20<br />
2 9<br />
8<br />
10<br />
HO 3 4 6 7<br />
OH<br />
4<br />
HO<br />
OO<br />
OH<br />
OH<br />
OH<br />
5<br />
O<br />
HO<br />
O<br />
O<br />
O<br />
HO<br />
HO<br />
OO<br />
OH<br />
OH<br />
OH<br />
6<br />
7<br />
OH<br />
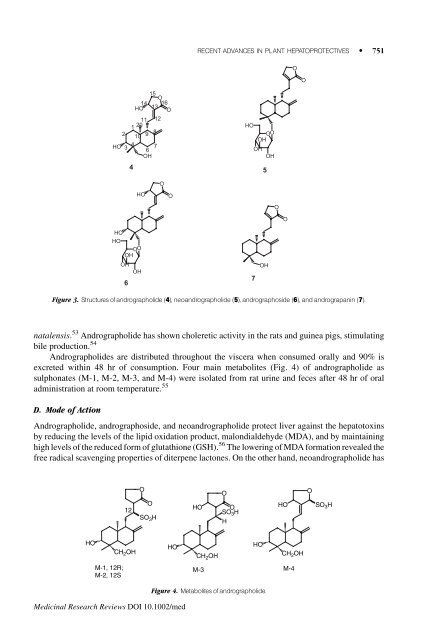
Figure 3. Structures of andrographolide (4), neoandrographolide (5), andrographoside (6), and andrograpan<strong>in</strong> (7).<br />
natalensis. 53 Andrographolide has shown choleretic activity <strong>in</strong> the rats and gu<strong>in</strong>ea pigs, stimulat<strong>in</strong>g<br />
bile production. 54<br />
Andrographolides are distributed throughout the viscera when consumed orally and 90% is<br />
excreted with<strong>in</strong> 48 hr of consumption. Four ma<strong>in</strong> metabolites (Fig. 4) of andrographolide as<br />
sulphonates (M-1, M-2, M-3, and M-4) were isolated from rat ur<strong>in</strong>e and feces after 48 hr of oral<br />
adm<strong>in</strong>istration at room temperature. 55<br />
D. Mode of Action<br />
Andrographolide, andrographoside, and neoandrographolide protect liver aga<strong>in</strong>st the hepatotox<strong>in</strong>s<br />
by reduc<strong>in</strong>g the levels of the lipid oxidation product, malondialdehyde (MDA), and by ma<strong>in</strong>ta<strong>in</strong><strong>in</strong>g<br />
high levels of the reduced form of glutathione (GSH). 56 The lower<strong>in</strong>g of MDA formation revealed the<br />
free radical scaveng<strong>in</strong>g properties of diterpene lactones. On the other hand, neoandrographolide has<br />
12<br />
O<br />
O<br />
SO 3 H<br />
HO<br />
O<br />
O<br />
SO 3 H<br />
H<br />
HO<br />
O<br />
SO 3 H<br />
HO<br />
M-1, 12R;<br />
M-2, 12S<br />
CH 2 OH<br />
HO<br />
HO<br />
CH 2 OH<br />
CH 2 OH<br />
M-3 M-4<br />
Medic<strong>in</strong>al Research Reviews DOI 10.1002/med<br />
Figure 4. Metabolites of andrographolide.