Mary Masterman - Oklahoma Biological Survey - University of ...
Mary Masterman - Oklahoma Biological Survey - University of ...
Mary Masterman - Oklahoma Biological Survey - University of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
3<br />
Introduction<br />
Objectives<br />
• To construct a Raman spectrograph system using a Raman head consisting <strong>of</strong> a set <strong>of</strong> filters and a sample holder,<br />
appropriate lenses, and an appropriate excitation laser, to be attached to a spectrograph.<br />
• To build a medium- to high-resolution inexpensive amateur Littrow spectrograph to be attached to the Raman head.<br />
• To test the Raman system with various Raman standards and make any modifications as necessary.<br />
Theory<br />
Raman spectroscopy, like infrared spectroscopy, is a form <strong>of</strong> vibrational spectroscopy. The Raman effect occurs when a photon<br />
striking a molecule excites an electron into a higher “virtual” energy level, and the electron decays back to a lower level, emitting a<br />
scattered photon (6). Unlike infrared spectroscopy, which results from a change in the dipole moment, Raman scattering results<br />
from changes in the polarizability <strong>of</strong> a molecule.<br />
In Raman, a high-intensity beam <strong>of</strong> light is directed to a sample. The vast majority <strong>of</strong> the light is scattered elastically (Rayleigh<br />
scattering) and the reflected light has the same wavelength as the incident light. A very small portion <strong>of</strong> the scattered radiation,<br />
however (on the order <strong>of</strong> 10^-7) is shifted to a different wavelength. The scattered photons are known as Raman scattering. A few<br />
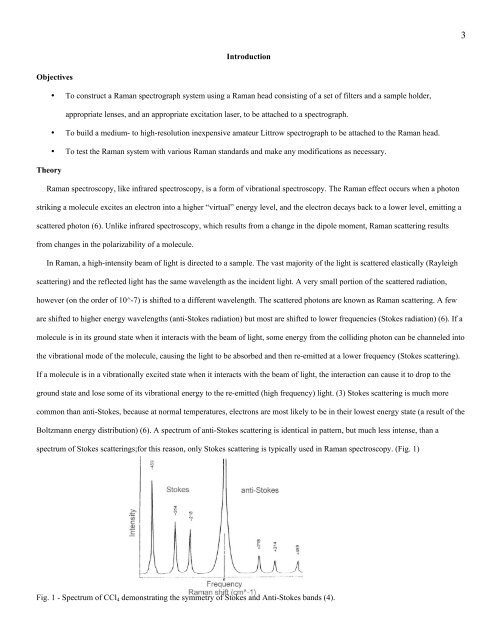
are shifted to higher energy wavelengths (anti-Stokes radiation) but most are shifted to lower frequencies (Stokes radiation) (6). If a<br />
molecule is in its ground state when it interacts with the beam <strong>of</strong> light, some energy from the colliding photon can be channeled into<br />
the vibrational mode <strong>of</strong> the molecule, causing the light to be absorbed and then re-emitted at a lower frequency (Stokes scattering).<br />
If a molecule is in a vibrationally excited state when it interacts with the beam <strong>of</strong> light, the interaction can cause it to drop to the<br />
ground state and lose some <strong>of</strong> its vibrational energy to the re-emitted (high frequency) light. (3) Stokes scattering is much more<br />
common than anti-Stokes, because at normal temperatures, electrons are most likely to be in their lowest energy state (a result <strong>of</strong> the<br />
Boltzmann energy distribution) (6). A spectrum <strong>of</strong> anti-Stokes scattering is identical in pattern, but much less intense, than a<br />
spectrum <strong>of</strong> Stokes scatterings;for this reason, only Stokes scattering is typically used in Raman spectroscopy. (Fig. 1)<br />
Fig. 1 - Spectrum <strong>of</strong> CCl 4 demonstrating the symmetry <strong>of</strong> Stokes and Anti-Stokes bands (4).