Thermal analysis and calorimetry solutions - Setaram
Thermal analysis and calorimetry solutions - Setaram
Thermal analysis and calorimetry solutions - Setaram
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
T h e r m a l a n a l y s i s a n d c a l o r i m e t r y<br />
s o l u t i o n s<br />
calvet
2 3
Process Safety<br />
Our Products<br />
The DSC131 evo is a highly robust <strong>and</strong> flexible DSC designed for busy laboratories<br />
with a wide range of applications <strong>and</strong> users. This entry level DSC features a unique<br />
<strong>and</strong> user changeable sensor, as well as a range of crucibles suitable for even the<br />
most energetic materials:<br />
<br />
<br />
<br />
<br />
The SENSYS evo is the premier DSC solution for Process Safety lab, the only DSC<br />
with a truly representative sample size - more than 10 times that of traditional DSC<br />
which allows for the accurate studies of heterogeneous mixtures <strong>and</strong> <strong>solutions</strong>.<br />
The unique Calvet detector also provides Cp measurements with precision of 1%.<br />
-120 to 830 (or 600)°C Temperature Range •<br />
<br />
<br />
<br />
<br />
Phi-Tec<br />
DSC 131 evo<br />
Sensys evo<br />
- 170 to 700 °C Temperature Range •<br />
0.01 to 100°C/min •<br />
+/- 0.5% calorimetric precision •<br />
30 & 100 µl crucibles, up to 500 bar (7500 psi) •<br />
0.01 to 30°C/min •<br />
+/- 0.1% calorimetric precision •<br />
Full autosampler option •<br />
120 to 320 µl crucibles, up to 500 bar (7250 psi) •<br />
Adiabatic Calorimetry is the final step in the design of<br />
a safe <strong>and</strong> efficient process <strong>and</strong> HEL provides both<br />
low <strong>and</strong> high Phi-Factor calorimeters which are critical<br />
for determining the heat <strong>and</strong> pressure evolved during<br />
uncontrolled runaway reactions. The NEW Phi-Tec I<br />
(a modern alternative to the classic Dow developed<br />
Accelerated Reaction Calorimeter) provides a unique<br />
bench-top system for optimal lab design.<br />
Both PHI-TEC I <strong>and</strong> II systems feature highly robust designs,<br />
including solid copper heaters, for long term operation <strong>and</strong> fast<br />
scan rates. The Phi-Tec II is a low Phi-Factor adiabatic calorimeter<br />
for simulation of true plant conditions <strong>and</strong> therefore precise<br />
calculations of vent sizes <strong>and</strong> emergency relief design.<br />
The C80 Calvet Calorimeter is one of the most<br />
powerful yet flexible tools available for the Process<br />
Safety laboratory. The C80 has a wide range<br />
of cells with a nominal volume of 12.5ml <strong>and</strong> as such<br />
can be used for truly representative samples, high<br />
precision Cp <strong>and</strong> also pressure measurement during<br />
decomposition. The range of cells includes ability<br />
to perform batch <strong>and</strong> semi-batch reactions under<br />
atmospheric pressure, applied pressure or for<br />
reactions that generate pressure. In scanning mode the<br />
C80 accurately asses chemical runaways. The range<br />
of sample cells include pressure up to 1000 bar (14600<br />
psi), high pressure mixing <strong>and</strong> even batch cells with a<br />
pressure transducer to measure evolved pressure.<br />
• Ambient to 300° °C Temperature Range<br />
• 0.001 to 2°C/min scanning rate<br />
• +/- 0.1% calorimetric precision<br />
• 350, 600 <strong>and</strong> 1000 bar (5075, 8700 <strong>and</strong> 14600 psi)<br />
pressure vessels<br />
• Mixing by membrane or reversal cell<br />
• High pressure reactions up to 200 bar (3000 psi)<br />
C80<br />
Advanced Kinetics Software from AKTS provides model free isoconversional<br />
kinetics for the precise modelling of runaway reactions.<br />
Using only 4 or 5 DSC or C80 signals recorded at different heating<br />
rates or at different isothermal temperatures a fully scaleable model<br />
of the decomposition is generated which is then used to predict<br />
such parameters as Time to Maximum Rate, SADT <strong>and</strong> stability in<br />
different thermal environments. Using AKTS software it is possible to<br />
use your adiabatic calorimeter as a single experiment verification, <strong>and</strong><br />
not a series of experiments at the start of a process evaluation.<br />
• Automatic baseline optimization <strong>and</strong> construction<br />
to DSC, nano <strong>and</strong> microDSC,<br />
C-80, DTA, HFC, TG, TG-MS<br />
• Model free kinetics : differential isoconversional<br />
(Friedman), integral isoconversional<br />
(Ozawa-Flynn-Wall), ASTM A698<br />
• Model fitting kinetics : all common kinetic<br />
models (i.e. autocatalytic <strong>and</strong> n-th order reactions (Fn),<br />
nucleation (Avrami-Erofeev, An), diffusion (Dn)…etc)<br />
AKTS<br />
• <strong>Thermal</strong> aging: prediction of reaction progress at any temperature profiles:<br />
isothermal, non-isothermal, stepwise,<br />
temperature modulation, NATO/STANAG 2895<br />
• <strong>Thermal</strong> safety: prediction of the reaction progress under adiabatic<br />
(TMR ad<br />
, thermal safety diagram <strong>and</strong> self heating rate curves) <strong>and</strong><br />
non-adiabatic conditions (Finite Element Analysis for critical design parameters:<br />
SADT, critical radius <strong>and</strong> hot discharge temperature, cook-off, etc.)<br />
4 5
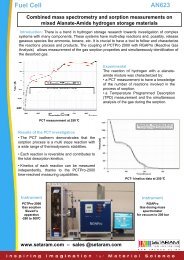
Applications<br />
DSC<br />
Customer Testimonial<br />
Screening DSC : Analysis of propergol<br />
The thermal decomposition of Propergol was<br />
studied in a tightly closed high pressure crucible <strong>and</strong><br />
in an open crucible with milligram-scale samples.<br />
Both experiments were run at a scanning rate of<br />
3°C/min, under inert gas conditions.<br />
It was observed that if the pressure rises during<br />
decomposition (closed crucibles), both total heat<br />
<strong>and</strong> reaction schemes are different. However,<br />
three peaks remain at similar positions <strong>and</strong> shape<br />
(cf. arrows), which was confirmed by built-in<br />
deconvolution module of Calisto data treatment<br />
software. The flexibility of SETARAM DSC’s allows<br />
gaining fast access to the behaviour under varying<br />
gaseous conditions (ex: ambient pressure vs. high<br />
pressure, oxidizing vs. inert conditions, etc…)<br />
“If I was setting up a new process safety laboratory, I would start<br />
with a Calvet C80 Calorimeter because, even if it can’t solve each<br />
process safety problem, it can be used for both synthesis reactions<br />
(mixing cell) <strong>and</strong> decomposition reactions, by offering an<br />
appreciable advantage, namely pressure measurement.<br />
I would start with a Calvet C80 Calorimeter, even if it meant<br />
supplementing it later on:<br />
- with a reaction calorimeter that would give a more representative<br />
measurement of the reactant accumulation <strong>and</strong> the heat release<br />
rate of the synthesis reaction.<br />
- with a DSC that offers greater screening productivity (scanning<br />
rate) <strong>and</strong> a wider temperature range (>300°C).”<br />
Prof. Francis STOESSEL,<br />
Swiss Institute for the Promotion of Safety, Basle, SWITZERLAND.<br />
Calvet 3D Calorimetry<br />
Adiabatic Calorimetry<br />
C80 : Analysis of diethyl sulfatel<br />
The thermal decomposition of diethyl<br />
sulfate was studied in a tightly closed high<br />
pressure cell equipped with a pressure<br />
measurement system.<br />
The experiment was run at a scanning rate<br />
of 0.5°C/min.<br />
It was observed that the biggest<br />
contribution to the pressure increase was<br />
due to a first decomposition at about 175°C.<br />
At the contrary, the second decomposition<br />
peak is probably consuming part of the<br />
gas produced at the first stage. More<br />
information can be extracted from<br />
this signal like pressure release rate,<br />
condensable / non condensable gas ratio.<br />
Phi-Tec<br />
This curve shows the decomposition<br />
reaction of 20% solution of N-nitroso-nmethyl-p-toluenesulfonamide<br />
(NMTS)<br />
in 1,4 Dioxane studied using Phi-Tec I<br />
<strong>and</strong> Phi-Tec II calorimeters.<br />
The decomposition was detected at<br />
71°C by Heat-Wait-Search method.<br />
Values of thermal inertia (Phi Factor)<br />
close to one (perfect adiabaticity) can<br />
be achieved with Phi-Tec II. It allows<br />
simulating true plant conditions, where<br />
heat accumulation strongly overcomes<br />
heat evacuation.<br />
6<br />
C80 : Polymerization study<br />
The polymerization reaction of Vinyl Pyrrolidone in<br />
presence of 4, 4’-azobis-cyanovaleric acid was<br />
studied using a C80 Calorimeter with membrane<br />
mixing cells.<br />
Pyrrolidone <strong>and</strong> 4, 4’-azobis-cyanovaleric acid were<br />
placed in the two chambers of the cell, separated by<br />
a thin membrane.<br />
The calorimeter was run under isothermal mode at<br />
50 °C. In-situ mixing was provided by piercing of the<br />
membrane.<br />
It was observed that a first, sharp peak, probably linked<br />
with the initiation of the reaction, was followed<br />
by a slower kinetics <strong>and</strong> higher heat process.<br />
This second peak is linked with the polymerization of<br />
Vinyl Pyrrolidone.<br />
Deconvolution of these peaks allows having access<br />
to the heat of the initiation, <strong>and</strong> by difference, the<br />
heat of polymerization could be calculated.<br />
Kinetics<br />
Advanced Kinetics<br />
(A) The decomposition of 3-methyl-4-<br />
nitrophenol was studied using DSC <strong>and</strong> C80.<br />
Heat flow (W/g)<br />
0.4 0.6 0.8 Heat flow (W/g)<br />
0.2<br />
Temperature (C°)<br />
Time (h)<br />
200 220 240 260 280 16 20 24 28 32<br />
0.2<br />
DSC<br />
Heat : -1,864.7 (J/g)<br />
A<br />
SADT<br />
360<br />
320<br />
Kinetics<br />
TMR 280<br />
ad 24h<br />
B<br />
240<br />
Φ=1<br />
Φ=3.2<br />
200<br />
D 160<br />
0<br />
180 200 220 240 260 280 0 5 10 15 20 25<br />
Temperature (C°)<br />
C<br />
Time (h)<br />
TMR ad 24h<br />
165<br />
160<br />
155<br />
150<br />
Temperature (C°) Temperature (C°)<br />
(B) The experiments at different<br />
heating rates were treated with<br />
AKTS - software.<br />
(C) The variation of the runaway<br />
time under true adiabatic mode<br />
(Phi Factor = 1) can be calculated<br />
for any process temperature.The<br />
critical value TMR ad<br />
= 24 hours is<br />
obtained at 153°C. Dashed lines<br />
depict the confidence interval.<br />
(D) An adiabatic experiment with<br />
a Phi factor = 3.2 was performed<br />
for the final validation of the<br />
simulation, <strong>and</strong> compared to<br />
adiabatic data. SADT can be<br />
determined applying “Finite<br />
Element Analysis”.<br />
7
Some International references<br />
Akzo Nobel - Netherl<strong>and</strong>s<br />
Aqura GmbH - Germany<br />
Ashl<strong>and</strong> Chemical Company - USA<br />
Astra Zeneca - Sweden, UK<br />
Bayer - Germany<br />
Boehringer - Germany<br />
Boehringer Ingelheim Chemicals - USA<br />
Boehringer Ingelheim Pharmaceuticals - USA<br />
Bristol-Myers Squibb - USA<br />
Centre d’études de Gramat - France<br />
ChemInform Saint-Petersbourg (CISP) – Russia<br />
Chimex - France<br />
Diosynth - Netherl<strong>and</strong>s<br />
DSM Nutritional Products AG - Switzerl<strong>and</strong><br />
Firmenich Inc - USA<br />
INERIS - France<br />
Institute of Safety <strong>and</strong> Security - Switzerl<strong>and</strong><br />
KOSHA - Korea<br />
LG Chem - Korea<br />
L’Oréal - France<br />
Merck - USA<br />
Mitsubishi Chemicals - Japan<br />
National Research Institute of Fire & Disaster - Japan<br />
NAVSEA (US Navy) - USA<br />
Nestec - Switzerl<strong>and</strong><br />
Novartis - Switzerl<strong>and</strong><br />
Organon - Netherl<strong>and</strong>s<br />
Oril Industrie, Groupe Servier - France<br />
Pfizer - UK, USA<br />
Sanofi Aventis - France<br />
Stazione Sperimentale per i Combustibili - Italy<br />
Syngenta - UK<br />
Synkem - France<br />
The Dow Chemical Company - USA<br />
Wacker Chemie - Germany<br />
Zambon - Italy<br />
Offices in United Kingdom, Germany,<br />
Italy, Switzerl<strong>and</strong> <strong>and</strong> Singapore<br />
w w w . s e t a r a m . c o m<br />
s a l e s @ s e t a r a m . c o m<br />
SETARAM INSTRUMENTATION<br />
7 rue de l’Oratoire<br />
69300 Caluire - France<br />
Phone +33(0)4 72 10 25 25<br />
Fax +33(0)4 78 28 63 55<br />
SETARAM Inc.<br />
8430 Central Ave. Suite 3D<br />
Newark, CA 94560 - USA<br />
Phone +1 (510) 793 3345<br />
Fax +1 (510) 402 4705<br />
SETARAM China<br />
Rm 1808, 618 Shangcheng Rd,<br />
Shanghai, 200120 - China<br />
Phone +86 21 50620017<br />
Fax +86 21 58797930<br />
PIRANA.net • Specifications are given as indications only <strong>and</strong> are not contractual • 09/09<br />
I n s p i r i n g I m a g i n a t i o n f o r M a t e r i a l S c i e n c e