Total Synthesis Highlights
Total Synthesis Highlights
Total Synthesis Highlights
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
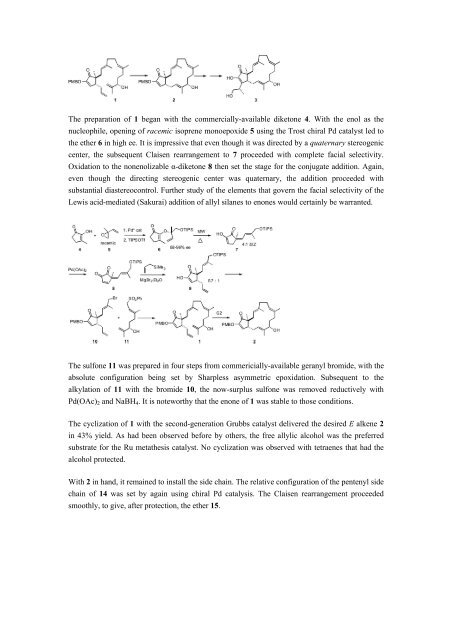
The preparation of 1 began with the commercially-available diketone 4. With the enol as the<br />
nucleophile, opening of racemic isoprene monoepoxide 5 using the Trost chiral Pd catalyst led to<br />
the ether 6 in high ee. It is impressive that even though it was directed by a quaternary stereogenic<br />
center, the subsequent Claisen rearrangement to 7 proceeded with complete facial selectivity.<br />
Oxidation to the nonenolizable α-diketone 8 then set the stage for the conjugate addition. Again,<br />
even though the directing stereogenic center was quaternary, the addition proceeded with<br />
substantial diastereocontrol. Further study of the elements that govern the facial selectivity of the<br />
Lewis acid-mediated (Sakurai) addition of allyl silanes to enones would certainly be warranted.<br />
The sulfone 11 was prepared in four steps from commericially-available geranyl bromide, with the<br />
absolute configuration being set by Sharpless asymmetric epoxidation. Subsequent to the<br />
alkylation of 11 with the bromide 10, the now-surplus sulfone was removed reductively with<br />
Pd(OAc) 2 and NaBH 4 . It is noteworthy that the enone of 1 was stable to those conditions.<br />
The cyclization of 1 with the second-generation Grubbs catalyst delivered the desired E alkene 2<br />
in 43% yield. As had been observed before by others, the free allylic alcohol was the preferred<br />
substrate for the Ru metathesis catalyst. No cyclization was observed with tetraenes that had the<br />
alcohol protected.<br />
With 2 in hand, it remained to install the side chain. The relative configuration of the pentenyl side<br />
chain of 14 was set by again using chiral Pd catalysis. The Claisen rearrangement proceeded<br />
smoothly, to give, after protection, the ether 15.