Total Synthesis Highlights
Total Synthesis Highlights
Total Synthesis Highlights
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
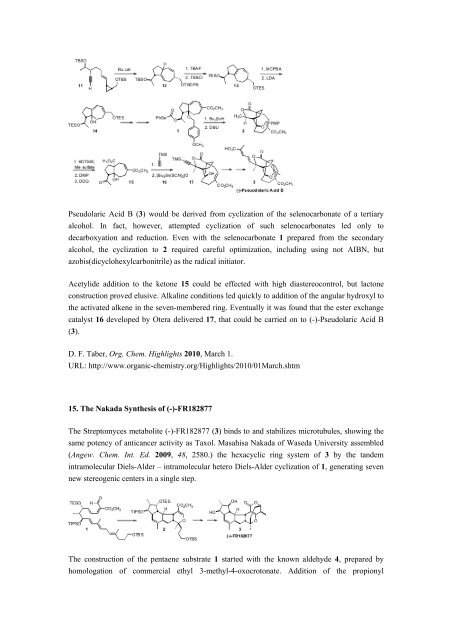
Pseudolaric Acid B (3) would be derived from cyclization of the selenocarbonate of a tertiary<br />
alcohol. In fact, however, attempted cyclization of such selenocarbonates led only to<br />
decarboxyation and reduction. Even with the selenocarbonate 1 prepared from the secondary<br />
alcohol, the cyclization to 2 required careful optimization, including using not AIBN, but<br />
azobis(dicyclohexylcarbonitrile) as the radical initiator.<br />
Acetylide addition to the ketone 15 could be effected with high diastereocontrol, but lactone<br />
construction proved elusive. Alkaline conditions led quickly to addition of the angular hydroxyl to<br />
the activated alkene in the seven-membered ring. Eventually it was found that the ester exchange<br />
catalyst 16 developed by Otera delivered 17, that could be carried on to (-)-Pseudolaric Acid B<br />
(3).<br />
D. F. Taber, Org. Chem. <strong>Highlights</strong> 2010, March 1.<br />
URL: http://www.organic-chemistry.org/<strong>Highlights</strong>/2010/01March.shtm<br />
15. The Nakada <strong>Synthesis</strong> of (-)-FR182877<br />
The Streptomyces metabolite (-)-FR182877 (3) binds to and stabilizes microtubules, showing the<br />
same potency of anticancer activity as Taxol. Masahisa Nakada of Waseda University assembled<br />
(Angew. Chem. Int. Ed. 2009, 48, 2580.) the hexacyclic ring system of 3 by the tandem<br />
intramolecular Diels-Alder – intramolecular hetero Diels-Alder cyclization of 1, generating seven<br />
new stereogenic centers in a single step.<br />
The construction of the pentaene substrate 1 started with the known aldehyde 4, prepared by<br />
homologation of commercial ethyl 3-methyl-4-oxocrotonate. Addition of the propionyl