A Route to Carbasugar Analogues - Jonathan Clayden - The ...
A Route to Carbasugar Analogues - Jonathan Clayden - The ... A Route to Carbasugar Analogues - Jonathan Clayden - The ...
Chapter 1: Introduction COR' i) ATPH, −78 °C then RM (2-3 eq) 1-12 hr ii) HCl (conc.) R 65a COR' + 65b COR' R Al Ph O Ph ATPH 3 entry RM R’ Solvent 65 / % 65a:b a 42 t-BuLi H PhMe/THF 81 99:1 b 42 n-BuLi H PhMe/THF 47 * 99:1 c 43 MeLi H PhMe 1 - d 45 e 45 OLi MeO H PhMe/THF 23 99:1 OLi MeO H PhMe/THF 77 99:1 f 44 PhMe 2 SiLi H PhMe 59 99:1 g 42 t-BuLi Me PhMe/THF 93 99:1 h 42 s-BuLi Me PhMe/THF 80 99:1 i 42 n-BuLi Me PhMe/THF 45 99:1 j 45 OLi MeO Me PhMe/THF 88 99:1 k 44 PhMe 2 SiLi Me PhMe 44 ** 6:1 l 43 MeLi Cl PhMe 99 2.6:1 m 43 PhLi Cl PhMe 96 99:1 n 43 i-PrMgBr Cl PhMe 71 13:1 o 43 t-BuMgBr Cl PhMe 90 15:1 p 43 OLi Cl PhMe 75 99:1 MeO q 44 PhMe 2 SiLi OMe PhMe 34 ‡ 35:13 * + 13% regiomeric 1,3-diene ** + 43% SM ‡+30% SM +35% rearomatised 65a Table 1.12 – selected dearomatisations by Yamamoto et al. Initial studies showed the reaction to be highly sensitive to the choice of solvent with a toluene-THF mixture being preferred at first, whilst more the recent work favours 37
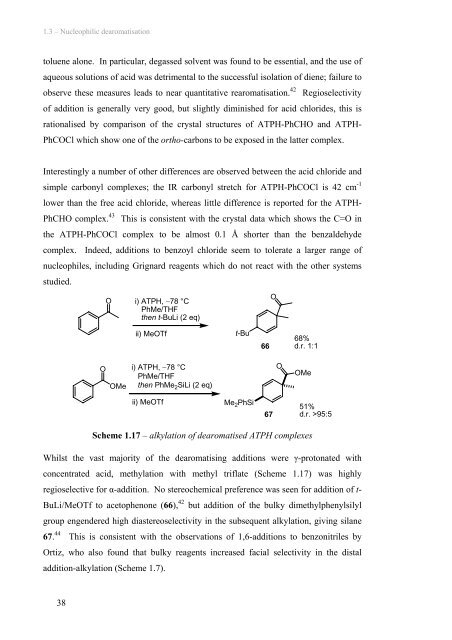
1.3 – Nucleophilic dearomatisation toluene alone. In particular, degassed solvent was found to be essential, and the use of aqueous solutions of acid was detrimental to the successful isolation of diene; failure to observe these measures leads to near quantitative rearomatisation. 42 Regioselectivity of addition is generally very good, but slightly diminished for acid chlorides, this is rationalised by comparison of the crystal structures of ATPH-PhCHO and ATPH- PhCOCl which show one of the ortho-carbons to be exposed in the latter complex. Interestingly a number of other differences are observed between the acid chloride and simple carbonyl complexes; the IR carbonyl stretch for ATPH-PhCOCl is 42 cm -1 lower than the free acid chloride, whereas little difference is reported for the ATPH- PhCHO complex. 43 This is consistent with the crystal data which shows the C=O in the ATPH-PhCOCl complex to be almost 0.1 Å shorter than the benzaldehyde complex. Indeed, additions to benzoyl chloride seem to tolerate a larger range of nucleophiles, including Grignard reagents which do not react with the other systems studied. O i) ATPH, −78 °C PhMe/THF then t-BuLi (2 eq) O ii) MeOTf t-Bu 66 68% d.r. 1:1 O OMe i) ATPH, −78 °C PhMe/THF then PhMe 2 SiLi (2 eq) O OMe ii) MeOTf Me 2 PhSi 67 51% d.r. >95:5 Scheme 1.17 – alkylation of dearomatised ATPH complexes Whilst the vast majority of the dearomatising additions were γ-protonated with concentrated acid, methylation with methyl triflate (Scheme 1.17) was highly regioselective for α-addition. No stereochemical preference was seen for addition of t- BuLi/MeOTf to acetophenone (66), 42 but addition of the bulky dimethylphenylsilyl group engendered high diastereoselectivity in the subsequent alkylation, giving silane 67. 44 This is consistent with the observations of 1,6-additions to benzonitriles by Ortiz, who also found that bulky reagents increased facial selectivity in the distal addition-alkylation (Scheme 1.7). 38
- Page 1 and 2: Dearomatising Addition of Organolit
- Page 3 and 4: 2.3 Synthetic Scope 64 2.3.1 Organo
- Page 5 and 6: Abstract Dearomatising Addition of
- Page 7 and 8: The Author The Author graduated fro
- Page 9 and 10: Abbreviations & Conventions Ac acet
- Page 11 and 12: RDS rate determining step R f RC rt
- Page 14 and 15: Chapter 1: Introduction Chapter 1 -
- Page 16 and 17: Chapter 1: Introduction controlled
- Page 18 and 19: Chapter 1: Introduction diastereome
- Page 20 and 21: Chapter 1: Introduction combine mor
- Page 22 and 23: Chapter 1: Introduction O Ar Cl TMP
- Page 24 and 25: Chapter 1: Introduction conditions,
- Page 26 and 27: Chapter 1: Introduction chiral oxaz
- Page 28 and 29: Chapter 1: Introduction Again a lar
- Page 30 and 31: Chapter 1: Introduction 34 Scheme 1
- Page 32 and 33: Chapter 1: Introduction The amino a
- Page 34 and 35: Chapter 1: Introduction It was envi
- Page 38 and 39: Chapter 1: Introduction O i) ATPH,
- Page 40 and 41: Chapter 1: Introduction 1.4 Nucleop
- Page 42 and 43: Chapter 1: Introduction Unlike the
- Page 44 and 45: Chapter 1: Introduction Diene (-)-8
- Page 46 and 47: Chapter 1: Introduction A scheme su
- Page 48 and 49: Chapter 1: Introduction Cl R + 93 S
- Page 50 and 51: Chapter 2 - Dearomatising additions
- Page 52 and 53: Chapter 2 - Dearomatising additions
- Page 54 and 55: Chapter 2 - Dearomatising additions
- Page 56 and 57: Chapter 2 - Dearomatising additions
- Page 58 and 59: Chapter 2 - Dearomatising additions
- Page 60 and 61: Chapter 2 - Dearomatising additions
- Page 62 and 63: Chapter 2 - Dearomatising additions
- Page 64 and 65: Chapter 2 - Dearomatising additions
- Page 66 and 67: Chapter 2 - Dearomatising additions
- Page 68 and 69: Chapter 2 - Dearomatising additions
- Page 70 and 71: Chapter 2 - Dearomatising additions
- Page 72 and 73: Chapter 2 - Dearomatising additions
- Page 74 and 75: Chapter 2 - Dearomatising additions
- Page 76 and 77: Chapter 2 - Dearomatising additions
- Page 78 and 79: Chapter 2 - Dearomatising additions
- Page 80 and 81: Chapter 2 - Dearomatising additions
- Page 82 and 83: Chapter 2 - Dearomatising additions
- Page 84 and 85: Chapter 2 - Dearomatising additions
1.3 – Nucleophilic dearomatisation<br />
<strong>to</strong>luene alone. In particular, degassed solvent was found <strong>to</strong> be essential, and the use of<br />
aqueous solutions of acid was detrimental <strong>to</strong> the successful isolation of diene; failure <strong>to</strong><br />
observe these measures leads <strong>to</strong> near quantitative rearomatisation. 42 Regioselectivity<br />
of addition is generally very good, but slightly diminished for acid chlorides, this is<br />
rationalised by comparison of the crystal structures of ATPH-PhCHO and ATPH-<br />
PhCOCl which show one of the ortho-carbons <strong>to</strong> be exposed in the latter complex.<br />
Interestingly a number of other differences are observed between the acid chloride and<br />
simple carbonyl complexes; the IR carbonyl stretch for ATPH-PhCOCl is 42 cm -1<br />
lower than the free acid chloride, whereas little difference is reported for the ATPH-<br />
PhCHO complex. 43 This is consistent with the crystal data which shows the C=O in<br />
the ATPH-PhCOCl complex <strong>to</strong> be almost 0.1 Å shorter than the benzaldehyde<br />
complex. Indeed, additions <strong>to</strong> benzoyl chloride seem <strong>to</strong> <strong>to</strong>lerate a larger range of<br />
nucleophiles, including Grignard reagents which do not react with the other systems<br />
studied.<br />
O<br />
i) ATPH, −78 °C<br />
PhMe/THF<br />
then t-BuLi (2 eq)<br />
O<br />
ii) MeOTf<br />
t-Bu<br />
66<br />
68%<br />
d.r. 1:1<br />
O<br />
OMe<br />
i) ATPH, −78 °C<br />
PhMe/THF<br />
then PhMe 2 SiLi (2 eq)<br />
O<br />
OMe<br />
ii) MeOTf<br />
Me 2 PhSi<br />
67<br />
51%<br />
d.r. >95:5<br />
Scheme 1.17 – alkylation of dearomatised ATPH complexes<br />
Whilst the vast majority of the dearomatising additions were γ-pro<strong>to</strong>nated with<br />
concentrated acid, methylation with methyl triflate (Scheme 1.17) was highly<br />
regioselective for α-addition. No stereochemical preference was seen for addition of t-<br />
BuLi/MeOTf <strong>to</strong> ace<strong>to</strong>phenone (66), 42 but addition of the bulky dimethylphenylsilyl<br />
group engendered high diastereoselectivity in the subsequent alkylation, giving silane<br />
67. 44 This is consistent with the observations of 1,6-additions <strong>to</strong> benzonitriles by<br />
Ortiz, who also found that bulky reagents increased facial selectivity in the distal<br />
addition-alkylation (Scheme 1.7).<br />
38