THE INFANCY OF SIGNAL TRANSDUCTION—GTP STIMULATION ...
THE INFANCY OF SIGNAL TRANSDUCTION—GTP STIMULATION ...
THE INFANCY OF SIGNAL TRANSDUCTION—GTP STIMULATION ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Classic Experiment<br />
13.1<br />
<strong>THE</strong> <strong>INFANCY</strong> <strong>OF</strong> <strong>SIGNAL</strong><br />
<strong>TRANSDUCTION—GTP</strong> <strong>STIMULATION</strong><br />
<strong>OF</strong> CAMP SYN<strong>THE</strong>SIS<br />
In the late 1960s the study of hormone action blossomed following the discovery<br />
that cyclic adenosine monophosphate (cAMP) functioned as a second messanger,<br />
coupling the hormone-mediated activation of a receptor to a cellular response. In<br />
setting up an experimental system to investigate the hormone induced synthesis of<br />
cAMP, Martin Rodbell discovered an important new player in intracellular signalling<br />
— guanosine triphosphate (GTP)<br />
Background<br />
The discovery of GTP’s role in regulating signal transduction<br />
began with studies on how glucagon and other hormones<br />
send a signal across the plasma membrane that<br />
eventually evokes a cellular response. At the outset of<br />
Rodbell’s studies, it was known binding of glucagon to<br />
specific receptor proteins embedded in the membrane<br />
stimulates production of cAMP. The formation of cAMP<br />
from ATP is catalyzed by a membrane bound enzyme<br />
called adenyl cyclase. It had been proposed that the action<br />
of glucagon, and other cAMP stimulating hormones,<br />
relied on additional molecular components that couple<br />
receptor activation to the production of cAMP. However,<br />
in studies with isolated fat cell membranes known as<br />
“ghosts,” Rodbell and his coworkers were unable to provide<br />
any further insight into how glucagon binding leads<br />
to an increase in production of cAMP. Rodbell then began<br />
a series of studies with a newly developed cell-free system,<br />
purified rat liver membranes, which retained both membrane-bound<br />
and membrane-associated proteins. These<br />
experiments eventually led to the finding that GTP is<br />
required for the glucagon-induced stimulation of adenyl<br />
cyclase.<br />
The Experiment<br />
One of Rodbell’s first goals was to characterize the binding<br />
of glucagon to the glucagon receptor in the cell-free rat<br />
liver membrane system. First, purified rat liver membranes<br />
were incubated with glucagon labeled with the radioactive<br />
isotope of iodine ( 125 I). Membranes were then separated<br />
from the unbound [ 125 I]glucagon by centrifugation. Once<br />
it was established that labeled glucagon would indeed<br />
bind to the purified rat liver cell membranes, the study<br />
went on to determine if this binding led directly to activation<br />
of adenyl cyclase and production of cAMP in the<br />
purified rat liver cell membranes.<br />
The production of cAMP in the cell-free system<br />
required the addition of ATP, the substrate for adenyl<br />
cyclase, Mg 2 , and an ATP-regenerating system consisting<br />
of creatine kinase and phosphocreatine. Surprisingly,<br />
when he glucagon binding experiment was repeated in the<br />
presence of these additional factors, Rodbell observed a<br />
50 percent decrease in glucagon binding. Full binding<br />
could be restored only when ATP was omitted from the<br />
reaction. This observation inspired an investigation of the<br />
effect of nucleoside triphosphates on the binding of<br />
glucagon to its receptor. It was shown that relatively high
(i.e., millimolar) concentrations of not only ATP but also<br />
uridine triphosphate (UTP) and cytidine triphosphate<br />
(CTP) reduced the binding of labeled glucagon. In contrast,<br />
the reduction of glucagon binding in the presence of<br />
GTP occurred at far lower (micromolar) concentrations.<br />
Moreover, low concentrations of GTP were found to stimulate<br />
the dissociation of bound glucagon from the receptor.<br />
Taken together, these studies suggested that GTP alters<br />
the glucagon receptor in a manner that lowers its affinity<br />
for glucagon. This decreased affinity both affects the ability<br />
of glucagon to bind to the receptor, and encourages the<br />
dissociation of bound glucagon.<br />
The observation that GTP was involved in the action<br />
of glucagon led to a second key question: Can GTP also<br />
exert an affect on adenyl cyclase? Addressing this question<br />
experimentally required the addition of both ATP, as a<br />
substrate for adenyl cyclase, and GTP, as the factor being<br />
examined, to the purified rat liver membranes. However,<br />
the previous study had shown that the concentration of<br />
ATP required as a substrate for adenyl cyclase could affect<br />
glucagon binding. Might it also stimulate adenyl cyclase?<br />
The concentration of ATP used in the experiment could<br />
not be reduced, because ATP was readily hydrolyzed by<br />
ATPases present in the rat liver membrane. To get around<br />
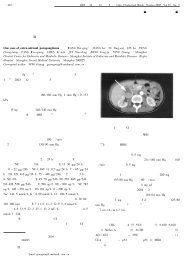
pmols cAMP<br />
1000<br />
Glucagon + GTP<br />
800<br />
600<br />
400<br />
Glucagon<br />
200<br />
Basal<br />
0 0<br />
5 10 15 20<br />
Minutes<br />
Effect of GTP on glucagon-stimulated cAMP production from<br />
AMP-PNP by purified rat liver membranes. In the absence of GTP,<br />
glucagon stimulates cAMP formation about twofold over the basal<br />
level in the absence of added hormone. When GTP also is added,<br />
cAMP production increases another fivefold. [Adapted from M.<br />
Rodbell et al., 1971, J. Biol. Chem. 246:1877.]<br />
this dilemma, Rodbell replaced ATP with an AMP analog,<br />
5¿<br />
-adenyl-imidodiphosphate (AMP-PNP), that can be converted<br />
to cAMP by adenyl cyclase, yet is resistant to<br />
hydrolysis by membrane ATPases. The critical experiment<br />
now could be performed. Purified rat liver membranes<br />
were treated with glucagon both in the presence and<br />
absence of GTP, and the production of cAMP from AMP-<br />
PNP was measured. The addition of GTP clearly stimulated<br />
the production of cAMP when compared to<br />
glucagon alone (see Figure) indicating that GTP promotes<br />
not only the binding of glucagon to its receptor but also<br />
the activation of adenyl cyclase.<br />
Discussion<br />
Two key factors led Rodbell and his colleagues to detect<br />
the role of GTP in signal transduction, whereas previous<br />
studies had failed to do so. First by switching from fat<br />
cell ghosts to the rat liver membrane system, the Rodbell<br />
researchers avoided contamination of their cell-free system<br />
with GTP, a problem associated with the procedure<br />
for isolating ghosts. Such contamination would mask the<br />
effects of GTP on glucagon binding and actviation of<br />
adenyl cyclase. Second, when ATP was first shown to<br />
influence glucagon binding, Rodbell did not simply accept<br />
the plausible explanation that ATP, the substrate for<br />
adenyl cyclase, also affects binding of glucagon. Instead,<br />
he chose to test the effects on binding of the other common<br />
nucleoside triphosphates. Rodbell later noted that he<br />
knew commercial preparations of ATP often are contaminated<br />
with low concentrations of other nucleoside triphosphates.<br />
The possibility of contamination suggested to him<br />
that small concentrations of GTP might exert large<br />
effects on glucagon binding and the stimulation of adenyl<br />
cyclase.<br />
This critical series of experiments stimulated a large<br />
number of studies on the role of GTP in hormone action,<br />
eventually leading to the discovery of G proteins, the<br />
GTP-binding proteins that couple certain receptors to the<br />
adenyl cyclase. Subsequently, an enormous family of<br />
receptors that require G proteins to transduce their signals<br />
were identified in eukaryotes from yeast to man. These G<br />
protein–coupled receptors are involved in action of many<br />
hormones as well as in a number of other biological activities<br />
including neurotransmission and the immune<br />
response. It is now known that binding of ligands to their<br />
cognate G protein–coupled receptors stimulates the associated<br />
G proteins to bind GTP. This binding causes transduction<br />
of a signal that stimulates adenyl cyclase to produce<br />
cAMP and also desensitization of the receptor, which<br />
then releases its ligand. Both of these affects were<br />
observed in Rodbell’s experiments on glucagon action. For<br />
these seminal observations, Rodbell was awarded the<br />
Nobel Prize for Physiology and Medicine in 1994.