PhD thesis
PhD thesis
PhD thesis
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Comparative neurogenesis, muscle<br />
development, and gene expression<br />
analyses in Brachiopoda<br />
<strong>PhD</strong> <strong>thesis</strong><br />
Andreas Altenburger
THE PHD SCHOOL OF SCIENCE<br />
FACULTY OF SCIENCE<br />
DEPARTMENT OF BIOLOGY<br />
UNIVERSITY OF COPENHAGEN<br />
DENMARK<br />
<strong>PhD</strong> <strong>thesis</strong><br />
Andreas Altenburger<br />
Comparative neurogenesis, muscle<br />
development, and gene expression analyses in<br />
Brachiopoda<br />
Principal supervisor<br />
Associate Prof. Dr. Andreas Wanninger<br />
Co-supervisor<br />
Prof. Dr. Pedro Martinez, University of Barcelona<br />
December , 2010
2 <br />
Principal supervisor<br />
Assoc. Prof. Dr. Andreas Wanninger<br />
Department of Biology<br />
Research Group for Comparative Zoology<br />
University of Copenhagen<br />
Copenhagen, Denmark<br />
Co-supervisor<br />
Prof. Dr. Pedro Martinez<br />
Department of Genetics<br />
University of Barcelona<br />
Barcelona, Spain<br />
Opponents<br />
Prof. Dr. Billie Swalla<br />
Department of Biology<br />
University of Washington<br />
Seattle, USA<br />
Prof. Dr. Bernard Degnan<br />
School of Biological Sciences<br />
The University of Queensland<br />
Brisbane, Australia<br />
Faculty opponent<br />
Assoc. Prof. Dr. Jørgen Olesen<br />
Zoological Museum<br />
Natural History Museum of Denmark<br />
Copenhagen, Denmark<br />
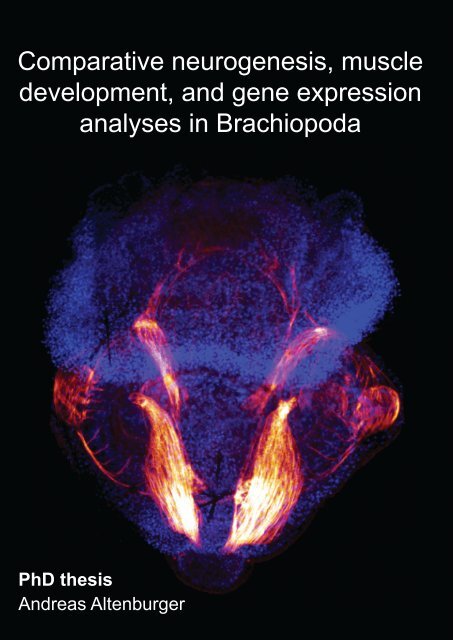
Cover legend<br />
Front: Myoanatomy of Joania (Argyrotheca) cordata. Maximum projection<br />
micrograph of a confocal laserscanning microscope stack. F-actin is labelled<br />
in red, cell nuclei are labelled in blue to indicate the outline of the specimen.<br />
Anterior faces upward and the specimen is approximately 280 µm long.<br />
Back: Schematic illustration of the specimen shown on front. The musculature<br />
comprises pedicle muscles (beige), longitudinal muscles (orange), central<br />
mantle muscles (brown), a U-shaped muscle (green), setae pouch muscles<br />
(red circles), circular mantle muscle (light blue), serial mantle muscles (dark<br />
orange), setae muscles (purple), apical longitudinal muscles (dark blue), and<br />
an apical transversal muscle (yellow).
<br />
3<br />
Content<br />
Preface ......................................................................................... 4<br />
Danish abstract ............................................................................... 5<br />
Abstract ......................................................................................... 6<br />
Short abstract ................................................................................. 7<br />
Acknowledgements ......................................................................... 8<br />
Chapter I ....................................................................................... 9<br />
Introduction ................................................................................ 9<br />
Brachiopoda .......................................................................... 9<br />
Nervous system .................................................................... 10<br />
Muscular system ................................................................... 10<br />
Gene expression ....................................................................11<br />
Material and methods ................................................................. 12<br />
Immunocytochemistry and phalloidin labeling .............................. 12<br />
Labeling of Pax3/7 proteins ..................................................... 12<br />
Detection of proliferating cells with BrdU (5-bromo-2-deoxyuridine)<br />
staining ............................................................................... 13<br />
Gene expression analyses ...................................................... 13<br />
Illustrations .......................................................................... 14<br />
Results and discussion ................................................................ 16<br />
Larval development ............................................................... 16<br />
Myogenesis ......................................................................... 20<br />
Neurogenesis with special focus on the apical organ of lophotrochozoan<br />
larvae ................................................................................. 20<br />
Distribution of Pax3/7 proteins in larvae of Terebratalia transversa 22<br />
Growth patterns of Terebratalia transversa ................................. 24<br />
Not and Cdx expression analyses ............................................. 26<br />
References ............................................................................... 28<br />
Chapter II ..................................................................................... 37<br />
Altenburger, A. & Wanninger, A. 2009 Comparative larval myogenesis<br />
and adult myoanatomy of the rhynchonelliform (articulate) brachiopods<br />
Argyrotheca cordata, A. cistellula, and Terebratalia transversa. Frontiers<br />
in Zoology 6: 1-14 ................................................................. 37<br />
Chapter III .................................................................................... 52<br />
Altenburger, A. & Wanninger, A. 2010 Neuromuscular development<br />
in Novocrania anomala: evidence for the presence of serotonin and a<br />
spiralian-like apical organ in lecithotrophic brachiopod larvae. Evolution<br />
& Development 12: 16-24 ....................................................... 52<br />
Chapter IV ................................................................................... 62<br />
Altenburger, A., Martinez, P. & Wanninger, A. First expression study of<br />
homeobox genes in Brachiopoda: the role of Not and Cdx in bodyplan<br />
patterning and germ layer specification. Submitted ...................... 62
4 <br />
Preface<br />
This <strong>thesis</strong> presents the results of three years of research at the University of<br />
Copenhagen from May 2007 until December 2010, including a research visit of<br />
one year at the University of Barcelona in 2009. The research on neurogenesis,<br />
myogenesis, and gene expression patterns in Brachiopoda was supervised by<br />
Assoc. Prof. Dr. Andreas Wanninger at the Research Group for Comparative<br />
Zoology, Department of Biology, University of Copenhagen, Denmark. The<br />
research on gene expression patterns was mainly carried out in the lab of Prof.<br />
Dr. Pedro Martinez, Department of Genetics, University of Barcelona, Spain.<br />
The <strong>PhD</strong> project was funded by The Danish Agency for Science, Technology<br />
and Innovation (grant no. 645-06-0294 to Andreas Wanninger).<br />
This project included several research visits of altogether nine weeks at the<br />
Sven Lovén Center for Marine Sciences in Kristineberg, Sweden, three weeks<br />
at the Moreton Bay Research Station on North Stradbroke Island, Australia,<br />
three weeks at the Banyuls-sur-mer Oceanological Observatory, France, and<br />
ten weeks at the Friday Harbor Laboratories, USA. Additional impact on my<br />
thinking about the field of evolution and development had the summer school on<br />
Evolution and Development of the Metazoans by Prof. Dr. Billie Swalla and Prof.<br />
Dr. Ken Halanych at the Friday Harbor Laboratories, University of Washington,<br />
USA, the Summer School on Evolutionary Developmental Biology by Prof. Dr.<br />
Alessandro Minelli and Assist. Prof. Giuseppe Fusco, University of Padua, Italy,<br />
and the EMBO course on Marine Animal Models in Evolution and Development<br />
organized by Prof. Dr. Detlev Arendt at the University of Gothenburg, Sweden.<br />
This <strong>thesis</strong> is composed of four chapters. Chapter I constitutes a short<br />
introduction to the research field and discusses the presented results in a<br />
broader perspective. Chapters II-IV contain two published papers and one<br />
submitted manuscript, which report the major findings made during this <strong>PhD</strong><br />
project.<br />
Copenhagen, December 2010<br />
Andreas Altenburger
<br />
5<br />
Danish abstract<br />
Brachiopoda udgør en dyrerække med en unik kropsbygning. Rækken omfatter<br />
ca. 370 nulevende arter opdelt i tre undergrupper, Rhynchonelliformea,<br />
Craniiformea og Linguliformea, men der er over 12.000 beskrevne fossile arter<br />
daterende helt tilbage til tidlig Kambrium. Der er uenighed om brachiopodernes<br />
fylogenetiske position som ofte debateres. Mit projekt har belyst dette problem<br />
gennem ny indsigt i brachipodernes ontogeni. Jeg har beskrevet udviklingen<br />
af nerve- og muskelsystemerne hos de rhynchonelliforme og craniiforme<br />
brachiopod larver af henholdsvis Terebratalia transversa og Novocrania<br />
anomala ved hjælp af immunohistokemiske indfarvninger kombineret med<br />
konfokal laserskanning mikroskopi og 3D-rekonstruktioner. Muskeldannelsen<br />
er beskrevet for både larver og voksne af Joania (Argyrotheca) cordata og<br />
Argyrotheca cistellula og ekspressionsmønstret af transskriptionsfaktorerne<br />
DP311, DP312 (Pax3/7) er beskrevet for larver og voksne af Terebratalia<br />
transversa. Ekspressionsmønstret af homeobox-generne TtrNot og TtrCdx er<br />
beskrevet for larver og juvenile af Terebratalia transversa ved hjælp af whole<br />
mount in situ hybridisering. De væsentligste resultater er: (1) Muskelanatomien<br />
hos rhynchonelliforme brachiopodlarver udviser stor lighed trods store forskelle i<br />
larvernes ydre morfologi. (2) Rhynchonelliforme og craniiforme brachiopodlarver<br />
af henholdsvis Terebratalia transversa og Novocrania anomala udviser et<br />
serotoninholdigt nervesystem, som omfatter fire eller otte flaskeformede celler<br />
i apikalorganet. Et sådant apikalorgan med flaskeformede celler er muligvis<br />
en morfologisk apomorfi for Lophotrochozoa. (3) Ekspressionsmønstret af<br />
TtrNot genet hos larverne af Terebratalia transversa indikerer en oprindelig<br />
funktion af dette gen i forbindelse med gastrulation, ektoderm specifikation<br />
og anlæggelse af nervebaner. For TtrCdx indikerer ekspressionsmønstret en<br />
oprindelig funktion i forbindelse med gastrulation samt dannelsen af den bageste<br />
del af det ektodermale væv hos Brachiopoda. Resultaterne bliver diskuteret<br />
i et fylogenetisk perspektiv gennem sammenligninger med andre rækker<br />
indenfor Lophotrochozoa, og implikationerne for evolutionen af Brachiopoda er<br />
fremhævet.
6 <br />
Abstract<br />
Brachiopods are a small phylum with a unique body plan comprising around<br />
370 living species and over 12.000 described fossil species dating back until the<br />
Lower Cambrian. The phylogenetic position of brachiopods is under controversial<br />
discussion. This project led to new insights into the ontogeny of brachiopods,<br />
which are divided into three clades, Rhynchonelliformea, Craniiformea,<br />
and Linguliformea. By use of immunocytochemistry combined with confocal<br />
laserscanning microscopy and 3D reconstruction software I describe the<br />
development of the nervous and muscular system in the rhynchonelliform and<br />
craniiform brachiopod larvae of Terebratalia transversa and Novocrania anomala.<br />
Myogenesis is described for larvae and adults of Joania (Argyrotheca) cordata<br />
and Argyrotheca cistellula and distribution of the transcription factor proteins<br />
DP311, DP312 (Pax3/7) for larvae and juveniles of Terebratalia transversa. The<br />
expression patterns of the developmental homeobox containing genes TtrNot<br />
and TtrCdx in larvae of Terebratalia transversa are described by use of whole<br />
mount in situ hybridization. The main results are: (1) The larval myoanatomy of<br />
rhynchonelliform brachiopod larvae is very similar, despite gross morphological<br />
differences in their outer morphology. (2) The rhynchonelliform and craniiform<br />
brachiopod larvae of Terebratalia transversa and Novocrania anomala show<br />
a serotonergic nervous system comprising eight or four flask-shaped cells<br />
in the apical organ. Such an apical organ with flask-shaped cells might be a<br />
morphological apomorphy of Lophotrochozoa. (3) The expression pattern of<br />
the TtrNot gene in larvae of Terebratalia transversa suggests an ancestral<br />
role of this gene in gastrulation and ectoderm specification in Brachiopoda.<br />
The expression pattern on TtrCdx suggests an ancestral role of this gene in<br />
gastrulation and the formation of posterior ectodermal tissue in Brachiopoda.<br />
The results are discussed in a phylogenetic framework compared to other<br />
lophotrochozoan phyla and implications of the results for the evolution of<br />
Brachiopoda are pointed out.
<br />
7<br />
Short abstract<br />
This <strong>thesis</strong> deals with selected aspects of brachiopod ontogeny. By use of<br />
immunocytochemistry combined with confocal laserscanning microscopy and<br />
3D reconstruction software the development of the nervous and muscular<br />
system of rhynchonelliform and craniiform brachiopod larvae is described. The<br />
expression patterns of the developmental homeobox containing genes TtrNot<br />
and TtrCdx are described by use of whole mount in situ hybridization. The main<br />
results are: (1) The larval myoanatomy of rhynchonelliform brachiopod larvae is<br />
similar despite gross morphological differences in their outer morphology. (2) The<br />
rhynchonelliform and craniiform brachiopod larvae show a serotonergic nervous<br />
system comprising eight or four flask-shaped cells in the apical organ. An apical<br />
organ comprising flask-shaped cells might be a morphological apomorphy of<br />
Lophotrochozoa. (3) The expression pattern of the TtrNot gene in larvae of<br />
Terebratalia transversa suggests an ancestral role of this gene in gastrulation<br />
and ectoderm specification in Brachiopoda. The expression pattern on TtrCdx<br />
suggests an ancestral role of this gene in gastrulation and the formation of<br />
posterior ectodermal tissue in Brachiopoda.
8 <br />
Acknowledgements<br />
The endeavour of such a <strong>thesis</strong> is impossible without the help of many people<br />
for whose support I am very grateful. Foremost I want to thank my principle<br />
supervisor Andreas Wanninger whose office door was always open and who<br />
did a great job in motivating and directing me towards the exciting parts of this<br />
study and especially the publication of the results.<br />
I am grateful to Pedro Martinez and his lab, namely Marta Chiodin, Amandine Bery,<br />
Eduardo Moreno, and Alexander Alsen for an inspiring time in Barcelona.<br />
I thank the teachers I had during <strong>PhD</strong> courses and who had a great influence on<br />
my thinking about the field of evo-devo, especially Billie Swalla, Ken Halanych,<br />
Alessandro Minelli, and Detlev Arendt.<br />
I thank the colleagues with whom I had the pleasure to share the room, lab,<br />
office, or a beer, Henrike Semmler, Nora Brinkmann, Tim Wollesen, Alen Kristof,<br />
Ricardo Neves, Julia Merkel, Birgit Meyer, Lennie Rotvit, Louise Würtz, Jan<br />
Bielecki, Jens Høeg, Lisbeth Haukrogh, Jan Lybeck, and visiting guests at the<br />
lab in Copenhagen.<br />
A special thank you to Anders Garm who translated the abstract into Danish.<br />
Many thanks go to the staff at the marine stations where I collected animals,<br />
in particular the Friday Harbor Laboratories, the Sven Lovén Centre for Marine<br />
Sciences, the Observatoire Océanologique de Banyuls-sur-mer, and the<br />
Moreton Bay Research Station.<br />
A special thank you goes to my wife Ruth who supported my work wherever<br />
she could and who took especially during the time in Barcelona the “burden” of<br />
caring full time almost alone for our son.<br />
This study was financially supported by a grant from the Danish Agency for<br />
Science, Technology and Innovation (grant no. 645-06-0294 to Andreas<br />
Wanninger) and a travel grant from Friday Harbor Labs to the author for<br />
participation in their summer course.
Introduction<br />
9<br />
Chapter I<br />
Introduction<br />
Brachiopoda<br />
The phylogenetic relationship of Brachiopoda is intensely debated among<br />
biologists and paleontologists alike. Brachiopods were already known by Linné,<br />
and 370 extant and more than 12.000 described fossil species are known (Linné<br />
1758; Ax 2003; Logan 2007). Brachiopods were significant members of the early<br />
Cambrian marine fauna and thus are one of the few phyla which are represented<br />
throughout the 550 million years of the Phanerozoic era, which extends from<br />
the first widespread appearance of organisms with mineralized skeletons until<br />
modern times (James et al. 1992). Historically, brachiopods have been assigned<br />
to different invertebrate groups, including molluscs (Lamarck 1801; Cuvier<br />
1805), bryozoans (Huxley 1853; Hancock 1858), bryozoans and phoronids<br />
(Hatschek 1888 ‘Tentaculata’; Hyman 1959 ‘Lophophorata’), or annelids (Morse<br />
1871). The three lophophorate groups or Brachiopoda alone have subsequently<br />
sometimes been regarded as deuterostomes (Brusca and Brusca 1990; Schram<br />
1991; Eernisse et al. 1992; Nielsen 1995). Since the appearance of molecular<br />
research tools, brachiopods have commonly been accepted to be protostomes<br />
(Field et al. 1988; Lake 1990; Halanych 1995; Hejnol et al. 2009). Brachiopod<br />
internal phylogeny distinguishes three clades; the inarticulate Linguliformea<br />
and Craniiformea and the articulate Rhynchonelliformea (Williams et al. 1996).<br />
Members of Linguliformea live buried in mud and have swimming juveniles<br />
instead of a true larval stage (Yatsu 1902). Members of craniiformea live with<br />
their ventral valve attached to stones and have two-lobed lecithotrophic larvae<br />
(Rowell 1960). Members of Rhynchonelliformea have a pedicle with which they<br />
attach themselves to rocks or other hard substrates (Williams et al. 1997). Their<br />
larvae have three lobes and are lecithotrophic (Freeman 2003). Traditionally,<br />
Linguliformea and Craniiformea have been grouped together as Inarticulata,<br />
while Rhynchonelliformea have been named Articulata because their valves<br />
are connected by a hinge (James et al. 1992).<br />
Brachiopods are certainly a comparatively minor phylum when only the number<br />
of recent species is considered. Nevertheless, they are present in all of the<br />
world’s oceans within all depth zones and the approximately 12.000 fossils<br />
species represent a rich source of paleontological information (Logan 2007).
10 Introduction<br />
Nervous system<br />
Microanatomical features related to the nervous system and the musculature of<br />
brachiopod larvae are virtually unknown. The literature on the nervous system<br />
of adult brachiopods boils down to descriptions by two authors on four species,<br />
Gryphus vitreus, Novocrania anomala, Discinisca lamellosa and Lingula anatina<br />
(van Bemmelen 1883; Blochmann 1892a, 1892b). Subsequent reviews of the<br />
same data are available from several authors (Helmcke 1939; Hyman 1959;<br />
Bullock and Horridge 1965a; Williams et al. 1997). In the rhynchonelliform<br />
brachiopod Gryphus vitreus the main body of nervous tissue is found around<br />
the esophagus and nerves emanate laterally from two ganglia, one subenteric<br />
ventral of the esophagus and one supraenteric dorsal of the esophagus<br />
(Rudwick 1970). The nervous system of brachiopod larvae or juveniles is<br />
only known for the linguliform Lingula anatina and Glottidia sp. and consists<br />
of a ventral lophophore system innervating the ciliary bands and a dorsal<br />
lophophore system innervating the body musculature (Hay-Schmidt 1992,<br />
2000). In order to fill the gap of knowledge concerning the brachiopod nervous<br />
system in rhynchonelliform and craniiform brachiopods, this study investigates<br />
the larval and juvenile neuroanatomy of Novocrania anomala (Craniiformea)<br />
and Terebratalia transversa (Rhynchonelliformea).<br />
Muscular system<br />
Adult brachiopods possess two main forms of muscular tissue. These are either<br />
bundles of muscle fibers that control the movement of the valves or myoepithelia<br />
in the lophophore (Williams et al. 1997). The muscles may be smooth, cross<br />
striated, or obliquely striated (Reed and Cloney 1977). Adult rhynchonelliform<br />
brachiopods comprise a pair of adductors, a pair of diductors, and a dorsal<br />
and a ventral pair of adjustor muscles that extend between the pedicle and the<br />
valves, moving the entire shell relative to the pedicle (Richardson and Watson<br />
1975). The adult craniiform Novocrania anomala comprises a pair of posterior<br />
as well as anterior adductors, a pair of oblique internal, and a pair of oblique<br />
lateral muscles (Bulman 1939). The muscular system of brachiopods and their<br />
larvae has been described by several authors (Hancock 1858; Kowalevski 1883;<br />
Blochmann 1892b; Helmcke 1939; Rudwick 1961; Reed and Cloney 1977), but<br />
no studies are available that use the benefit of up-to-date techniques such as<br />
immunocytochemistry in combination with confocal laserscanning microscopy<br />
and 3D reconstruction software in order to visualize in detail the more cryptic<br />
muscle sets of larval and adult brachiopods. Investigation of myogenesis was<br />
carried out in the course of the present <strong>PhD</strong> study in order to obtain a clearer<br />
picture of the entire brachiopod muscular bauplan as well as the dynamics of
Introduction<br />
11<br />
muscular remodeling during metamorphosis using the following species: Joania<br />
cordata (previously Argyrotheca cordata), Argyrotheca cistellula, Novocrania<br />
anomala, and Terebratalia transversa.<br />
Gene expression<br />
Data on the molecular processes that regulate animal development have<br />
greatly expanded within recent years (Carroll 2005). The investigation of gene<br />
families that encode signaling molecules with roles in the control of cell fate<br />
specification, proliferation, movement, and segment polarity has considerably<br />
improved our understanding of metazoan ontogeny (Davidson and Levine<br />
2008). So far, only few sequences of developmental genes have been<br />
identified in brachiopods, such as members of the Wnt gene family (Holland<br />
et al. 1991) and Hox genes (de Rosa et al. 1999), but nothing has so far been<br />
published on the expression of these genes during ontogeny. This might not<br />
be too surprising, since marine animals as little accessible as brachiopods are<br />
unlikely to be favored as candidate model organisms for this kind of studies<br />
(Sommer 2009). However, since the bauplan of some brachiopods has not<br />
changed significantly since the Early Cambrian, gene expression data from this<br />
phylum are very interesting because they may shed light on gene functions in<br />
the brachiopod ancestor. This information might contribute to understand the<br />
evolution of early bilaterian animals. In this study, the expression patterns of<br />
the developmental homeobox genes Not and Cdx were investigated in larvae<br />
of the rhynchonelliform brachiopod Terebratalia transversa. This was done in<br />
order to reveal the functions of these genes in Brachiopoda and to assess their<br />
ancestral function in animal development.<br />
Not is a homeobox gene and representatives of its family play an important role<br />
during notochord formation in vertebrates (Abdelkhalek et al. 2004). Its role in<br />
invertebrate development is not well known (Martinelli and Spring 2004). Cdx<br />
is a homeobox gene that is expressed in posterior tissues of almost all phyla<br />
investigated so far (Hejnol and Martindale 2008). In addition to the posterior<br />
tissues it was found to be expressed in mesoderm, gut, brain, and the central<br />
nervous system of mice, lancelets, and annelids, as well as in the gut of<br />
Drosophila and the mesoderm of Artemia (Macdonald and Struhl 1986; Duprey<br />
et al. 1988; Brooke et al. 1998; Copf et al. 2004; Fröbius and Seaver 2006). The<br />
gene expression patterns presented in this <strong>thesis</strong> are the first of their kind for<br />
the phylum Brachiopoda.
12 Material and methods<br />
Material and methods<br />
Immunocytochemistry and phalloidin labeling<br />
A range of morphological and molecular methods were applied to representative<br />
species of two main groups of Brachiopoda: Rhynchonelliformea and<br />
Craniiformea. The musculature was investigated by use of fluorescent<br />
conjugated phalloidin. Phalloidin is a toxin found in the mushroom Amanita<br />
phalloides and it binds irreversibly to F-actin.<br />
The antibodies applied to stain the nervous system bind specifically to neurotransmitters<br />
such as serotonin (5-Hydroxytryptamine [5 HT]), neuropeptides<br />
such as FMRFamide, or tubulins such as α-tubulin.<br />
An overview of the species investigated, the methods, and the antibodies<br />
applied is given in Table 1.<br />
Labeling of Pax3/7 proteins<br />
Arthropods and annelids generate new body segments from a posterior growth<br />
zone (Anderson 1973; Meier 1984; Scholtz and Dohle 1996). It has been<br />
proposed that the situation in Brachiopoda is comparable to the segmented<br />
Annelida (Morse 1871). The larval lobes in rhynchonelliform brachiopods<br />
suggest a segmented body plan and a segmented worm like ancestor of<br />
Brachiopoda (Morse 1873). In order to investigate if the rhynchonelliform<br />
brachiopod larvae of Terebratalia transversa show remnants of segmentation<br />
from a potentially segmented ancestor, the larvae were stained with antibodies<br />
that bind specifically on proteins of the Pax3/7 gene family.<br />
The antibodies DP311 and DP312 detect domains of the Pax 3/7 and non-Pax3/7<br />
proteins in Drosophila and Schistocerca (grasshopper) embryos (Davis et al.<br />
2005). The monoclonal antibodies were raised in mouse and made available<br />
by Michalis Averof (Institute of Molecular Biology & Biotechnology, Greece).<br />
DP311 stains the following proteins in Drosophila: paired (prd), gooseberry<br />
(gsb), gooseberry-neuro (gsbn), aristaless, homeobrain, and repo. DP312<br />
stains prd, gsb, gsbn and Rx.<br />
Larvae and juveniles of Terebratalia transversa were collected and fixed as<br />
described in Chapter II. The primary antibodies were used in a concentration of<br />
1:30 and the staining was applied as described in Chapters II and III. The stained<br />
specimens were analyzed with a Leica DM RXE 6 TL fluorescence microscope<br />
equipped with a TCS SP2 AOBS laserscanning device (Leica Microsystems,<br />
Wetzlar, Germany).
Material and methods<br />
13<br />
Detection of proliferating cells with BrdU (5-bromo-2-deoxyuridine)<br />
staining<br />
BrdU labeling was carried out, in order to identify possible growth zones in<br />
rhynchonelliform brachiopod larvae. BrdU is incorporated into the DNA of<br />
proliferating cells during the S-phase of the cell cycle. Staining of BrdU thus<br />
allows for visualization of dividing cells and their progenies. Larvae of Terebratalia<br />
transversa of the following developmental stages: 6, 11, 24, 35, 48, 60, and 96<br />
hours after fertilization (hpf) were incubated in 0.1mM BrdU (Sigma-Aldrich,<br />
St. Louis, MO, USA) in seawater at 11.5ºC for 6 – 48h. In another experiment<br />
larvae were cultured in 10mM BrdU in seawater for 30 min and subsequently<br />
the larvae were cultured in BrdU free seawater (pulse-chase experiment). After<br />
the treatment with BrdU the larvae were fixed in 4% paraformaldehyde in PBS<br />
for 1 hour at room temperature and then treated for 10 min at 37ºC in 0.01mg/<br />
ml proteinase K in PBS. After that they were kept for 10 min in 0.1N HCl on<br />
ice, 1 hour at 37ºC in 2N HCl, 1 hour in PBS with three changes, and 15 min in<br />
PBT (PBS with Tween 20). Then, the larvae were incubated in 1:500 mouseanti-BrdU<br />
antibody in PBT over night at 4 ºC, washed for 1 hour in PBS with<br />
three changes, 1 hour in 1:200 diluted TRITC, and finally 1 hour in PBS with<br />
three changes. Stained larvae were mounted in glycerol and analyzed with a<br />
Leica DM RXE 6 TL fluorescence microscope equipped with a TCS SP2 AOBS<br />
laserscanning device (Leica Microsystems, Wetzlar, Germany).<br />
Gene expression analyses<br />
The expression of developmental genes was studied by whole mount in<br />
situ hybridization (WMISH). Thereby, target mRNA is visualized with a<br />
complementary RNA probe which contains DIG labelled uridine (Digoxigenin-<br />
11-uridine-5’-triphosphate). The digoxigenin is subsequently stained with a<br />
Anti-DIG-AP, fab fragments antibody that contains alkaline phosphatase (AP)<br />
which in turn is made visible by a reaction with BCIP (5-Bromo-4-chloro-3-<br />
indolyl phosphate) and NBT (nitro blue tetrazolium chloride). In this reaction<br />
BCIP is dephosphorylated by AP and dimerizes to leucoindigo. This dimer is<br />
then oxidized by NBT to an insoluable dark blue 5,5’-dibromo-4,4’ precipitate<br />
(Trinh et al. 2007). The precipitate is visible in daylight conditions and also<br />
reflects laser light which allows the use of this technique in combination with a<br />
confocal laserscanning microscope (Jekely and Arendt 2007).<br />
There are several WMISH protocols available which usually have to be adapted<br />
to the organism they are intended for. Protocols developed for several species<br />
were tested in this study, namely one for the sea urchin Strongylocentrotus
14 Material and methods<br />
purpuratus, the cnidarian Nematostella vectensis, and the polychaete Platynereis<br />
dumerilii, respectively (Arendt et al. 2001; Long and Rebagliati 2002; Martindale<br />
et al. 2004; Venuti et al. 2004). The N. vectensis protocol was found to be the<br />
best of the tested protocols for the brachiopod Terebratalia transversa and was<br />
used accordingly to investigate the expression patterns of TtrNot and TtrCdx<br />
(Chapter IV).<br />
Illustrations<br />
Illustrations were done with Photoshop CS3 and Illustrator CS3 software<br />
(Adobe, San Jose, CA, USA).
<br />
15<br />
Table 1. List of species investigated, methods applied, and antibodies used. (+) indicates positive<br />
results, (-) indicates that no clear signal could be obtained, 5HT – stains nervous tissue, ad –<br />
adult, BrdU – 5-bromo-2-deoxyuridine (stains proliferating cells), CLSM – confocal laserscanning<br />
microscopy, DAPI – (stains nucleic acids), engrailed – labels segment boundaries in Drosophila,<br />
Immunostar – producer of antibodies, juv – juvenile, Pax 3/7 – labels segment boundaries in<br />
Drosophila, Phalloidin – stains F-actin, Sigma – Sigma-Aldrich, producer of antibodies, Tubulin<br />
– stains cilia and nervous tissue, WMISH – whole mount in situ hybridization.<br />
Clade<br />
Species<br />
Stages<br />
investigated<br />
larval juv ad<br />
Method<br />
applied<br />
Antibodies<br />
applied<br />
(signal + or -)<br />
Chapter<br />
Rhynchonelliformea<br />
Joania<br />
(Argyrotheca)<br />
cordata<br />
+ - + CLSM<br />
5 HT (Sigma) (-)<br />
DAPI (+)<br />
FMRF (-)<br />
Phalloidin (+)<br />
Tubulin (+)<br />
II<br />
Rhynchonelliformea<br />
5 HT (Sigma) (-)<br />
Argyrotheca<br />
+ - + CLSM<br />
FMRF (-)<br />
II<br />
cistellula<br />
Phalloidin (+)<br />
5 HT<br />
(Immunostar) (+)<br />
BrdU (+)<br />
Cdx (+)<br />
Rhynchonelliformea<br />
Terebratalia<br />
transversa<br />
+ + -<br />
CLSM<br />
WMISH<br />
DAPI (+)<br />
Engrailed (-)<br />
FMRF (-)<br />
I, II, IV<br />
Not (+)<br />
Pax 3/7 (+)<br />
Phalloidin (+)<br />
Tubulin (+)<br />
Phalloidin (+)<br />
Craniiformea<br />
5 HT<br />
Novocrania<br />
+ + - CLSM<br />
(Immunostar) (+)<br />
III<br />
anomala<br />
Tubulin (+)<br />
FMRF (-)
16 Results and discussion<br />
Results and discussion<br />
Larval development<br />
Terebratalia transversa, a representative of Rhynchonelliformea<br />
Larval development of Terebratalia transversa and regional specification during<br />
embryogenesis has been described previously (Freeman 1993). My results<br />
are congruent with these data. The oocyte (Fig. 1A) divides approximately 2<br />
hours after fertilization (hpf) at a water temperature of 11.5 °C and two polar<br />
bodies are formed (Fig. 1B). Cleavage is radial and the first two cleavages are<br />
holoblastic (Fig. 1B, C). The early blastula is composed of rounded cells (Fig.<br />
1D) and gastrulation occurs approximately at 19 hpf (Fig. 1E). In the gastrula,<br />
the wall of the archenteron forms contact with the cells of the ectoderm, i.e.,<br />
the blastocoel virtually disappears (Fig. 1F). Later in development the gastrula<br />
elongates and the blastopore becomes slit-like elongated (Fig. 1G). The three<br />
larval lobes start to form as the embryo elongates further and an apical tuft<br />
appears, which is lost later in development (Fig 1H, I). At this stage the larvae<br />
become positively phototactic and usually swim in the upper part of the water<br />
column. At approximately 75 hpf the larvae are almost fully developed and the<br />
apical, mantle, and pedicle lobe are formed. Only the setae continue to grow<br />
at this point of development. The fully developed larvae eventually become<br />
negatively phototactic. Then, they swim towards the bottom of the culture dish<br />
and repeatedly touch the surface with their apical lobe, probably in order to test<br />
if the substrate is suitable for metamorphosis. Larvae settle and metamorphose<br />
between 120 and 300 hpf. The juveniles still retain the larval setae and the<br />
lophophore starts to form after settlement (Fig. 1J). Metamorphosis appears to<br />
be catastrophic since all tissues seem to be reformed during metamorphosis<br />
(Stricker and Reed 1985a, 1985b).
Results and discussion<br />
17<br />
A B C<br />
D<br />
0 2 3 10<br />
at<br />
E F ec G<br />
H<br />
AL<br />
AL<br />
en<br />
* *<br />
*<br />
18 24<br />
30 36<br />
I<br />
se<br />
AL<br />
ML<br />
PL<br />
J<br />
se<br />
se<br />
se<br />
Lo<br />
75 hpf Pe 360 hpm<br />
se<br />
Figure 1. Developmental stages of Terebratalia transversa at a water temperature of 11.5 °C.<br />
Numbers indicate the age in hours after fertilization (hpf) for all stages except of J where it is<br />
hours after the onset of metamorphosis (hpm). Size of all stages is around 120 µm in diameter,<br />
except for J where it is around 200 µm. Anterior is oriented upwards and cilia are omitted for<br />
clarity. (A) unfertilized oocyte (black) with an egg shell (grey). (B) Lateral view of two cell stage<br />
with two polar bodies and the egg shell (grey). (C) Apical view of a four cell stage. (D) Sagittal<br />
section through an early blastula. (E) Sagittal section through a late blastula at the onset of<br />
gastrulation. (F) Gastrula with ectoderm (ec), endoderm (en), and blastopore (asterisk). The<br />
gastrula starts to swim at this point of development. (G) Elongated late gastrula with slit-like<br />
blastopore (asterisk) and first signs of a distinguished apical lobe (AL). (H) Larva with further<br />
developed lobes, almost closed blastopore (asterisk), and apical tuft (at). (I) Fully established<br />
larva with apical lobe (AL), mantle lobe (ML), and pedicle lobe (PL). Four sets of setae bundles<br />
(se, only two visible) originate from the mantle lobe. (J) Juvenile with lophophore (Lo), and<br />
pedicle (Pe). The remaining larval setae (se) extend beyond the two valves.<br />
se<br />
Novocrania anomala, a representative of Craniiformea<br />
Development of Novocrania anomala and regional specification during<br />
embryogenesis has been described previously (Nielsen 1991; Freeman 2000).<br />
My results are congruent with these data. However, the two authors disagree<br />
about the development of the coelom and the formation of the mesoderm.<br />
According to Nielsen, the sheet of cells that invaginates during gastrulation is<br />
composed of two cell populations, endoderm and mesoderm, whereas Freeman<br />
states that the mesoderm is formed by individual cells which immigrate from the<br />
endodermal cell layer after invagination has been completed (Nielsen 1991;<br />
Freeman 2000). Nielsen describes the coelom as consisting of an anterior<br />
coelomic pouch in the apical lobe and three pairs of coelomic cavities in the
18 Results and discussion<br />
posterior lobe of the larva, whereas Freeman denies the existence of larval<br />
coelomic structures and states that the coelom develops after the larvae have<br />
undergone metamorphosis (Nielsen 1991; Freeman 2000). The methods used<br />
here do not allow a conclusive statement concerning coelom and mesoderm<br />
formation in larvae of N. anomala, there is more work needed to resolve the<br />
controversies on an ultrastructural level.<br />
Cleavage is radial and the first two divisions are holoblastic (Fig. 2B). The gastrula<br />
is first spherical and invagination takes place at the vegetal pole of the larva.<br />
The archenteron cells come to lie opposite of the ectoderm. Subsequently, the<br />
blastocoel disappears completely (Fig. 2C). Later in development the gastrula<br />
elongates and the blastopore comes to lie at the postero-ventral side of the<br />
swimming larva (Fig. 2D). The elongated gastrula subsequently differentiates<br />
into two larval lobes, an apical lobe and a posterior lobe (Fig. 2E, F). Larval<br />
development completes with the growth of three pairs of dorsal setal bundles<br />
on the posterior lobe (Fig. 2G). Prior to settlement, the larva swims along the<br />
bottom of the culture dish, probably in order to test if the substrate is suitable<br />
for settlement. In contrast to the descriptions by Nielsen (1991), the larvae do<br />
not curl before metamorphosis. Although curled larvae are found in the culture<br />
dishes, these seem to be unable to metamorphose. What causes the curling<br />
is unclear, however it can clearly be seen in the musculature of settled larvae<br />
that the remaining larval muscles are elongated and relaxed in contrast to the<br />
contracted musculature of curled larvae (Fig. 3A, B, and Chapter III).<br />
At a water temperature of 14 °C, metamorphosis takes place around six to ten<br />
days after fertilization (dpf). During metamorphosis the larva attaches to the<br />
substrate, secretes the shell, and retains its larval lobes, which are subsequently<br />
transformed and form the lophophore and other adult organs (Figs. 2H, 3B,<br />
C).
Results and discussion<br />
19<br />
A B C D<br />
0 4<br />
25 * 32<br />
E F G H<br />
AL<br />
se<br />
se<br />
se<br />
se<br />
AL<br />
PL<br />
40 72 105<br />
se<br />
AL<br />
PL<br />
se<br />
se<br />
ec<br />
en<br />
*<br />
se<br />
se<br />
se<br />
se<br />
se<br />
s<br />
AL<br />
PL<br />
ec<br />
en<br />
se<br />
se<br />
200<br />
Figure 2. Developmental stages of Novocrania anomala at a water temperature of 14 °C.<br />
Numbers indicate the age in hours after fertilization (hpf) for all stages except for H where it is<br />
hours after the onset of metamorphosis (hpm). Size of all stages is around 130 µm in diameter.<br />
Anterior is oriented upwards. Cilia have been omitted for clarity (A) Unfertilized oocyte (black)<br />
with egg shell (grey). (B) Apical view of a four cell stage with the egg shell at 4hpf. (C) Frontal<br />
view of a gastrula with blastopore (asterisk), ectoderm (ec), and endoderm (en). The gastrula<br />
starts to swim at this point of development. (D) Lateral view of an elongated gastrula with<br />
ectoderm (ec) and endoderm (en). The blastopore (asterisk) is situated on the posterior end<br />
of the gastrula. (E) Dorsal view of an elongated gastrula with almost distinct apical lobe (AL).<br />
(F) Ventral view of an early two-lobed larva with apical lobe (AL) and posterior lobe (PL). The<br />
blastopore is closed and larval setae (se) start to grow on the posterior side. (G) Dorsal view of<br />
a fully developed larva with apical lobe (AL), posterior lobe (PL), and three pairs of dorsal setae<br />
bundles (se). (H) Ventral view of a juvenile after metamorphosis. The larval apical lobe (AL) and<br />
pedicle lobe (PL) are still visible. The juvenile shell (s) is formed on the dorsal side with larval<br />
setae (se) extending from it.<br />
Figure 3. Metamorphosis of Novocrania anomala. Scale bars equal 50 µm, anterior is up. A and<br />
B are overlays of confocal maximum projections of phalloidin stainings and light micrographs.<br />
C is a light micrograph of a live specimen. (A) Ventral view of a curled larva with contracted<br />
musculature (empty arrow), apical lobe (AL), and posterior lobe (PL). (B) Musculature of a<br />
settled juvenile with remaining elongated larval musculature (empty arrowheads), juvenile<br />
anterior adductor muscles (aad), larval setae pouch muscles (arrows), larval anterior lobe (AL),<br />
posterior lobe (PL), and juvenile shell (s). (C) Dorsal view of a settled juvenile with remaining<br />
larval setae (se), shell (s), posterior lobe (PL), and apical lobe (AL) which has started to form<br />
the lophophore (Lo).
20 Results and discussion<br />
Myogenesis<br />
Results of larval myogenesis and adult myoanatomy are presented in Chapters<br />
II and III.<br />
Actin and myosin are molecules present in all metazoans including basal groups<br />
such as sponges and Trichoplax (Thiemann and Ruthmann 1989; Kanzawa et<br />
al. 1995). It has been proposed that the basal pattern of musculature in the<br />
bilaterian ancestor was a grid of outer circular and inner longitudinal musculature,<br />
the Hautmuskelschlauch (HMS), which has in some taxa been modified in<br />
combination with the evolution of hard exoskeletons (Schmidt-Rhaesa 2007a).<br />
Brachiopods have discrete bundles of muscle fibers that control the movement<br />
of the valves and the tentacles. Brachiopods have further myoepithelia which<br />
are found on the inner side of coelomic epithelia, in the parietal bands, in mantle<br />
lobes, and in the lophophore (Williams et al. 1997). Additionally, I could show<br />
that adults of the species Joania cordata, Argyrotheca cistellula, Novocrania<br />
anomala, and Terebratalia transversa contain discrete bundles of mantle<br />
retractor muscles (Chapters II, III), a character that is probably present in all<br />
brachiopods.<br />
The larval musculature is similar among the rhynchonelliform brachiopods<br />
investigated herein (Chapter II). Remnants of a HMS could not be distinguished.<br />
Accordingly, if the ancestor of Brachiopoda had a HMS, it was lost during the<br />
evolution of this phylum. Interestingly, the larval musculature of the craniiform<br />
brachiopod Novocrania anomala is very different from the musculature of<br />
the investigated rhynchonelliform brachiopod larvae (Chapter III). This hints<br />
towards an early split in the evolution of these two groups. This is confirmed by<br />
the fossil record, which estimates the split between the rhynchonelliform and<br />
craniiform clade to have taken place before the Ordovician 485 million years<br />
ago (Freeman and Lundelius 2005).<br />
Neurogenesis with special focus on the apical organ of<br />
lophotrochozoan larvae<br />
Results on neurogenesis in brachiopod larvae and juveniles are presented in<br />
Chapters III and IV.<br />
Adult rhynchonelliform brachiopods have a nervous system which is concentrated<br />
around the esophagus and comprises two ganglia, one dorsal and one ventral of<br />
the esophagus, as well as circumenteric nerves that innervate the lophophore,<br />
ventral mantle nerves, and dorsal mantle nerves (van Bemmelen 1883; Bullock<br />
and Horridge 1965a). The nervous system of adult Novocrania anomala lacks<br />
the dorsal ganglion. The circumenteric nerves emanate laterally from the ventral
Results and discussion<br />
21<br />
ganglion and form a ring around the esophagus. Additional lateral and brachial<br />
nerves emanate from the ventral ganglion (Blochmann 1892b). The nervous<br />
system of the lecithotrophic rhynchonelliform brachiopod larvae of Terebratalia<br />
transversa comprises two sets of four serotonergic flask-shaped cells in the<br />
apical organ that are connected by neurites to a larval neuropil in the apical lobe<br />
(Chapter IV). The nervous system of the lecithotrophic craniiform brachiopod<br />
larvae of Novocrania anomala comprises four centrally positioned serotonergic<br />
flask-shaped cells in the apical organ connected to two ventral nerve cords that<br />
extend ventrolaterally along the body (Chapter III). Linguliform planktotrophic<br />
brachiopod juveniles of Lingula anatina and Glottidia sp. possess a nervous<br />
system comprising an apical ganglion as well as dorsal and ventral lophophore<br />
nerves (Hay-Schmidt 1992). The apical ganglion of Glottidia sp. contains<br />
numerous serotonergic cells that are associated with two serotonergic tracts<br />
which project into the ciliary band (Hay-Schmidt 2000). This system is probably<br />
not homologous to the apical organs found in T. transversa and N. anomala,<br />
since there are numerous serotonergic cells in Glottidia sp. and none of these<br />
cells are flask-shaped.<br />
The evolution of nervous systems has been reviewed by several authors<br />
(Bullock and Horridge 1965b; Holland 2003; Schmidt-Rhaesa 2007b; Arendt<br />
et al. 2008; Benito-Gutiérrez and Arendt 2009; Wanninger 2009; Harzsch and<br />
Wanninger 2010). All eumetazoans are able to transmit information between<br />
cells. Sponges use electric signals albeit lacking neurons (Leys et al. 1999),<br />
cnidarians have a nerve net with electrical and chemical synapses (Anderson<br />
and Trapido-Rosenthal 2009), and bilaterians have a nervous system that often<br />
comprises some sort of “brain” and nerve cords or neurite bundles (Rieger et al.<br />
2010). The last common ancestor of cnidarians and bilaterians most likely had<br />
a nerve net which developed under the control of anteroposterior patterning<br />
genes (Westfall 1996; Westfall and Elliott 2002; Watanabe et al. 2009). The<br />
question whether the ancestor of Protostomia and Deuterostomia had a diffuse<br />
nervous system or a centralized nervous system is still hotly debated and a<br />
final statement can not yet be made (Younossi-Hartenstein et al. 1997; Arendt<br />
and Nübler-Jung 1999; Holland 2003; Lowe et al. 2003; 2006; Telford 2007;<br />
De Robertis 2008; Reichert 2009; Harzsch and Wanninger 2010). Recent<br />
studies showed that larval Entoprocta and adult Mollusca show a tetraneurous<br />
condition consisting of one pair of ventral and on pair of more dorsally positioned<br />
lateral nerve cords. In addition, the creeping-type entoproct larva and the<br />
polyplacophoran larvae exhibit a complex apical organ consisting of around<br />
eight centrally positioned serotonergic flask-shaped cells which are surrounded<br />
by several peripheral cells. (Wanninger et al. 2007; Fuchs and Wanninger 2008;
22 Results and discussion<br />
Wanninger 2008; 2009). In Nemertea, the lecithotrophic, non-pilidium like larva<br />
of Quasitetrastemma stimpsoni shows a pair of serotonergic flask-shaped cells<br />
in the apical organ plus a pair of subapical cells and two posterior neurons<br />
that are located ventrolaterally (Chernyshev and Magarlamov 2010). Annelid<br />
larvae show a serotonergic apical organ comprising up to four cells. The apical<br />
organ is associated with the prototrochal nerve ring which in turn is connected<br />
to two ventral nerve cords (Voronezhskaya et al. 2003; McDougall et al. 2006;<br />
Brinkmann and Wanninger 2008). The apical organ of ectoproct cyphonautes<br />
larvae comprises two pairs of serotonergic cell bodies from which lateral nerves<br />
project towards the corona (Hay-Schmidt 2000; Gruhl 2009). One of the two cell<br />
clusters in the apical organ contains flask-shaped cells (Nielsen and Worsaae<br />
2010). In the apical organ of the ectoproct coronate larva of Bugula neritina<br />
two flask-shaped serotonergic cells are present (Pires and Woollacott 1997;<br />
Shimizu et al. 2000). In the actinotroch larva of Phoronida, the apical organ<br />
contains numerous serotonergic cells, but these are probably not flask-shaped<br />
(Santagata 2002; Santagata and Zimmer 2002; Wanninger 2008).<br />
Taken together, the data that have recently become available on lophotrochozoan<br />
larval neuroanatomy suggest that an apical organ comprising serotonergic<br />
flask-shaped cells was present in larvae of the last common lophotrochozoan<br />
ancestor (Wanninger 2008). Accordingly, an apical organ containing such cells<br />
might be a morphological apomorphy of Lophotrochozoa.<br />
Distribution of Pax3/7 proteins in larvae of Terebratalia transversa<br />
A sister group relationship of Brachiopoda with Annelida has been hypothesized<br />
based on molecular data as well as on paleontological data and is supported by<br />
the notion that annelids and brachiopods share similarities in the ultrastructure<br />
of their setae (Gustus and Cloney 1972; Orrhage 1973; Field et al. 1988; Lake<br />
1990; Conway Morris and Peel 1995; Lüter 2000b). Several developmental<br />
genes that are involved in the establishment of segments and segmentation in<br />
animals have been characterized, some of which belong to the Pax3/7 group.<br />
Pax3 and Pax7 genes probably arose by duplication from unique ancestral Pax3/7<br />
genes and have similarities in their protein sequence and expression (Hayashi et<br />
al. 2010). Pax3/7 genes are also known as Pax group III genes and include the<br />
pair-rule gene paired (prd), the segment polarity genes gooseberry (gsb), and<br />
gooseberry-neuro (gsbn), a gene that is expressed in the developing nervous<br />
system and, together with engrailed, establishes the posterior commissures in<br />
the fruit fly Drosophila melanogaster (Noll 1993; Colomb et al. 2008). Together<br />
with their vertebrate homologs (Pax-3 and Pax-7) the Pax3/7 group forms one
Results and discussion<br />
23<br />
of four classically defined subgroups of the Pax family transcription factors<br />
(Balczarek et al. 1997). Pax3/7 shares its expression among distantly related<br />
insects and shows several patterns including pair-rule, segment polarity,<br />
and neural patterning (Davis et al. 2005). In crustaceans Pax3/7 genes are<br />
expressed in iterated stripes (Davis et al. 2005). In myriapods and chelicerates<br />
Pax3/7 gene expression exhibits iterated stripes that form early in the posteriormost<br />
part of the germ band (Davis et al. 2005). In the tardigrade Hypsibius<br />
dujardini, the Pax3/7 proteins localize in a segmentally iterated pattern in the<br />
ectoderm, after establishment of endomesoderm segmentation, but before the<br />
visible segmentation of the ectoderm (Gabriel and Goldstein 2007). Pax3/7 is<br />
also localized within the developing head region of the tardigrade embryo, but<br />
no pair-rule pattern is visible during any stage of embryogenesis (Gabriel and<br />
Goldstein 2007). Tardigrades, together with arthropods and onychophorans<br />
belong to Panarthropoda (Halanych 2004).The expression pattern of Pax3/7 in<br />
H. dujardini suggests that the pair-rule function of Pax3/7 may have arisen near<br />
the base of Arthropoda.<br />
In the annelid Platynereis dumerilii Pax3/7 proteins are found in the peripheral<br />
nervous system (Kerner et al. 2009). In larvae of the brachiopod Terebratalia<br />
transversa DP311 and DP312 show identical staining patterns. Pax 3/7 starts<br />
to be present in four cells of the apical lobe in the late elongated gastrula (Fig.<br />
4B). The cells containing Pax3/7 products are later distributed in a ring on the<br />
apical lobe of early three-lobed larvae without setae (Fig. 4C). Fully established<br />
larvae show a loose distribution of cells that contain Pax3/7 products in their<br />
apical lobe (Fig. 4D, E). In juveniles Pax3/7 containing cells are mainly found<br />
in the growing lophophore (Fig. 4F). The presence of Pax3/7 gene products<br />
in the apical lobe indicates a function of those genes during neurogenesis in<br />
T. transversa. However, further experiments are necessary in order to assess<br />
whether the staining specifically shows Pax3/7 protein products, since the<br />
antibodies used were developed against the Pax3/7 sequences of Drosophila<br />
melanogaster. Ideally, cloning of the sequences of the Pax3/7 homologs of<br />
Terebratalia transversa should be carried out, followed by mapping of the<br />
epitopes of DP311 and DP312 on peptide arrays with the known peptide<br />
sequences of T. transversa and other metazoans (Harlow and Lane 1999). The<br />
final proof would then be in situ hybridizations with the specific corresponding<br />
probes. In addition, a double staining with serotonin would be necessary in<br />
order to prove that the cells containing Pax3/7 gene products are co-localized<br />
with the nervous system.
24 Results and discussion<br />
Growth patterns of Terebratalia transversa<br />
Figure 4. Staining of<br />
Pax3/7 proteins with<br />
DP311. Overlay of confocal<br />
maximum projections on<br />
light micrographs. Anterior<br />
is up and scale bars equal<br />
50 µm. (A) Gastrula with<br />
blastopore (asterisk) and<br />
no signal. (B) Late gastrula<br />
with slit-like blastopore<br />
(asterisk). Pax3/7 proteins<br />
are stained in four cells<br />
in the future apical lobe<br />
(al). (C) Early three-lobed<br />
larva with almost closed<br />
blastopore (asterisk).<br />
Pax3/7 proteins are present<br />
in several cells of the apical<br />
lobe (al) and distributed in<br />
a ring around it. No signal<br />
is found in the mantle lobe<br />
(ml) and in the pedicle lobe<br />
(pl) (D) Lateral view of a<br />
larva with apical lobe (al),<br />
mantle lobe (ml), pedicle<br />
lobe (pl), and setae (se).<br />
Pax3/7 protein containing<br />
cells are concentrated in<br />
the dorsal part of the apical<br />
lobe. (E) Fully established<br />
larva with apical lobe (al),<br />
mantle lobe (ml), pedicle<br />
lobe (pl), and setae (se).<br />
Cells with Pax3/7 proteins<br />
are loosely distributed in<br />
the apical lobe. (F) Juvenile<br />
after metamorphosis.<br />
Pax3/7 proteins are loosely<br />
expressed in the developing<br />
lophophore (Lo) of the<br />
juvenile. The dorsal shell (s)<br />
of this specimen is slightly<br />
shifted upwards relative<br />
to its natural position, and<br />
larval setae (se) extend out<br />
of the valves<br />
In order to identify possible growth zones in brachiopod larvae, proliferating<br />
cells in Terebratalia transversa were labeled with 5-bromo-2-deoxyuridine<br />
(BrdU). Dividing cells are equally distributed in the blastula stage (Fig. 5A),<br />
the gastrula (Fig. 5B), and the elongated gastrula (Fig. 5C). In the elongated<br />
gastrula, cells divide mostly in the center of the larva and form the mantle lobe,<br />
which is marked by a ring of dividing cells (Fig. 5D). Thereafter, dividing cells<br />
are again equally distributed throughout the larva (Fig. 5E). Larvae competent<br />
for metamorphosis also show an equal distribution of proliferating cells after a<br />
pulse-chase experiment, which once again indicates that there are no distinct<br />
growth zones that form most parts of the larval body, but that dividing cells are<br />
found throughout the developing specimen (Fig. 5F). The BrdU data suggest<br />
that from the viewpoint of proliferation zones, there are no similarities between
Results and discussion<br />
25<br />
Figure 5. Pattern of<br />
BrdU staining in larvae of<br />
Terebratalia transversa.<br />
Overlay of confocal<br />
maximum projections and<br />
light micrographs. Scale<br />
bars equal 50 µm. All stages<br />
show an equal distribution<br />
of proliferating cells,<br />
there are thus no distinct<br />
growth zones identifiable.<br />
(A) Blastula. (B) Early<br />
gastrula with blastopore<br />
(asterisk). (C) Late slightly<br />
elongated gastrula with<br />
blastopore (asterisk). (D)<br />
Early three lobed stage<br />
with the developing apical<br />
lobe (al), mantle lobe (ml),<br />
and pedicle lobe (pe). (E)<br />
Three lobed stage with<br />
apical lobe (al), mantle<br />
lobe (ml), and pedicle lobe<br />
(pl). This stage is at the<br />
onset of setae formation.<br />
(F) Fully developed threelobed<br />
stage with apical<br />
lobe (al), mantle lobe (ml),<br />
and pedicle lobe (pl).<br />
the development of Annelida and Brachiopoda. For annelids, it has been shown<br />
that, although the post-metamorphic segments originate from a posterior growth<br />
zone, the precise location of the growth zone can vary (Seaver et al. 2005;<br />
Brinkmann and Wanninger 2010). However, the rhynchonelliform brachiopods<br />
are regarded derived amongst brachiopod subgroups (Carlson 1995). The<br />
distribution of proliferating cells in Terebratalia transversa can therefore not<br />
completely rule out the possibility that the brachiopod ancestor had a growth<br />
zone. Similar experiments in linguliform and craniiform brachiopods are needed<br />
in order to further assess this issue.<br />
The Annelida-Brachiopoda sister group hypo<strong>thesis</strong> based on the ultrastructure<br />
of the setae has been questioned by Lüter who showed that there is a difference<br />
in the ultrastructure of larval and adult setae in the brachiopods Lingula anatina,<br />
Notosaria nigricans, and Calloria inconspicua, suggesting a convergent<br />
evolution of setae in Annelida and Brachiopoda (Lüter 2000b). An additional
26 Results and discussion<br />
argument against segmentation in brachiopod larvae is that the segmented<br />
appearance with three larval lobes is not recognizable by the inner bauplan<br />
on the ultrastructural level (Lüter 2000a). This has been shown for Notosaria<br />
nigricans and Calloria inconspicua. In these species, a single coelomic anlage<br />
forms one compartment with all mesodermally derived cells separated only by<br />
cellular membranes. Thus, there is only one mesoderm compartment in these<br />
larvae, which encloses one coelomic cavity (Lüter 2000a). In the segmented<br />
Annelida the coelom forms one pair of coelomic cavities in each segment<br />
(Anderson 1973).<br />
Not and Cdx expression analyses<br />
Results of gene expression patterns of the homeobox genes TtrNot and TtrCdx<br />
are presented in Chapter IV.<br />
In Terebratalia transversa, the ortholog of the homeobox gene Not, TtrNot, is<br />
expressed in the ectoderm from the beginning of gastrulation until completion<br />
of larval development, which is marked by a three-lobed body with larval setae.<br />
Expression starts at gastrulation in two areas lateral to the blastopore and<br />
subsequently extends over the animal pole of the gastrula. With elongation of<br />
the gastrula, expression at the animal pole narrows to a small band, whereas<br />
the areas lateral to the blastopore shift slightly towards the future anterior region<br />
of the larva. Upon formation of the three larval body lobes, TtrNot expressing<br />
cells are present only in the posterior part of the apical lobe. Expression ceases<br />
entirely at the onset of larval setae formation. TtrNot expression is absent in<br />
unfertilized eggs, in embryos prior to gastrulation, and in settled individuals<br />
during and after metamorphosis. Comparison to the expression patterns of Not<br />
genes in other metazoan phyla suggests an ancestral role in gastrulation, germ<br />
layer (ectoderm) specification, and neural patterning, with co-opted functions in<br />
notochord formation in chordates and left/right determination in ambulacrarians<br />
and vertebrates (Chapter IV).<br />
In Terebratalia transversa the ParaHox gene TtrCdx is expressed on the<br />
posterior side of the blastopore and its expression stays in this region until the<br />
three-lobed larva is fully formed. The expression of TtrCdx suggests a function<br />
of this gene during gastrulation and ectoderm patterning in Brachiopoda. The<br />
pattern of Cdx in other metazoans ranges from expression in the mesoderm,<br />
gut, brain, central nervous system to posterior tissues (Fröbius and Seaver<br />
2006). The basal function of Cdx is probably in patterning of posterior tissues.
General conclusions and perspectives for future research<br />
27<br />
General conclusions and perspectives for future research<br />
The results presented herein are the first developmental gene expression<br />
studies in Brachiopoda, as well as the first detailed comparative description of<br />
myogenesis and neurogenesis in brachiopod larvae based on antibody staining,<br />
confocal laserscanning microscopy, and 3D reconstruction software. This study<br />
shows that microanatomical data can yield new insights into the evolution and<br />
development of lesser known metazoan phyla such as Brachiopoda. It provides<br />
the first evidence of an apical organ in brachiopod larvae that comprises<br />
serotonergic flask-shaped cells, similar to those found in ectoprocts and<br />
spiralians. This result strongly suggests that such an apical organ constitutes a<br />
morphological apomorphy of Lophotrochozoa.<br />
Gene expression analyses of TtrNot imply an ancestral role of this gene in<br />
gastrulation and ectoderm specification in Brachiopoda. The function of Not in<br />
notochord formation in chordates and left/right determination in ambulacrarians<br />
and vertebrates might thus be co-opted in these deuterostome clades. Analysis<br />
of the TtrCdx gene expression suggests an ancestral role in gastrulation and the<br />
formation of posterior tissues in Brachiopoda as well as in Bilateria in general.<br />
Further studies should extend the database of brachiopod morphogenesis<br />
and gene expression patterns to more organ systems as well as to the third<br />
brachiopod subtaxon, Linguliformea. This would allow for a full representation<br />
of the phylum Brachiopoda with its three clades Craniiformea, Linguliformea,<br />
and Rhynchonelliformea and should allow significant inferences concerning<br />
gene function and organ system evolution within this lophophorate phylum.<br />
Such data would allow insights into the evolution of organ systems, and body<br />
plans in Brachiopoda. Additionally, investigation of gene expression patterns in<br />
Brachiopoda is needed in order to compare the function of genes, co-option,<br />
and ancestral gene functions among Brachiopoda and other animal phyla. An<br />
expressed sequence tags or genome-based approach would be the best choice<br />
in order to obtain the sequences of the whole range of developmental genes.<br />
Preferably, this should be done for one representative of each brachiopod clade.<br />
Morphological and molecular data together would facilitate the reconstruction of<br />
the evolution of organ systems in Brachiopoda once the phylogenetic position<br />
of Brachiopoda and its sister groups has been settled.
28 References<br />
References<br />
Abdelkhalek, H. B., Beckers, A., Schuster-Gossler, K., Pavlova, M. N.,<br />
Burkhardt, H., Lickert, H., Rossant, J., Reinhardt, R., Schalkwyk, L. C.,<br />
Müller, I., Herrmann, B. G., Ceolin, M., Rivera-Pomar, R., and Gossler, A.<br />
2004. The mouse homeobox gene Not is required for caudal notochord<br />
development and affected by the truncate mutation. Genes Dev 18:1725-<br />
1736.<br />
Anderson, D. 1973. Embryology and Phylogeny in Annelids and Arthropods.<br />
Oxford: Pergamon Press Ltd.<br />
Anderson, P. and Trapido-Rosenthal, H. 2009. Physiological and chemical<br />
analysis of neurotransmitter candidates at a fast excitatory synapse in<br />
the jellyfish Cyanea capillata (Cnidaria, Scyphozoa). Invert Neurosci<br />
9:167-173.<br />
Arendt, D., Denes, A. S., Jékely, G., and Tessmar-Raible, K. 2008. The evolution<br />
of nervous system centralization. Philos Trans R Soc Lond B Biol Sci<br />
363:1523-1528.<br />
Arendt, D. and Nübler-Jung, K. 1999. Comparison of early nerve cord<br />
development in insects and vertebrates. Development 126:2309-2325.<br />
Arendt, D., Technau, U., and Wittbrodt, J. 2001. Evolution of the bilaterian larval<br />
foregut. Nature 409:81-85.<br />
Ax, P. 2003. Multicellular animals: Order in nature - system made by man. Vol.<br />
III. Heidelberg: Springer.<br />
Balczarek, K. A., Lai, Z. C., and Kumar, S. 1997. Evolution of functional<br />
diversification of the paired box (Pax) DNA-binding domains. Mol Biol<br />
Evol 14:829-842.<br />
Benito-Gutiérrez, È. and Arendt, D. 2009. CNS evolution: New insight from the<br />
mud. Curr Biol 19:R640-R642.<br />
Blochmann, F. 1892a. Ueber die Anatomie und die verwandtschaftlichen<br />
Beziehungen der Brachiopoden. Arch. Freunde Naturgesch. Mecklenbg.<br />
46:37-50.<br />
Blochmann, F. 1892b. Untersuchungen über den Bau der Brachiopoden. Jena:<br />
Gustav Fischer.<br />
Brinkmann, N. and Wanninger, A. 2008. Larval neurogenesis in Sabellaria<br />
alveolata reveals plasticity in polychaete neural patterning. Evol Dev<br />
10:606-618.<br />
Brinkmann, N. and Wanninger, A. 2010. Integrative analysis of polychaete<br />
ontogeny: cell proliferation patterns and myogenesis in trochophore<br />
larvae of Sabellaria alveolata. Evol Dev 12:5-15.
References<br />
29<br />
Brooke, N. M., Garcia-Fernandez, J., and Holland, P. W. H. 1998. The ParaHox<br />
gene cluster is an evolutionary sister of the Hox gene cluster. Nature<br />
392:920-922.<br />
Brusca, R. C. and Brusca, G. J. 1990. Invertebrates: Sunderland, Mass: Sinauer<br />
Associates.<br />
Bullock, T. H. and Horridge, G. A. 1965a. Lophophorate phyla: Ectoprocta,<br />
Brachiopoda, and Phoronida. In Structure and function in the nervous<br />
system of invertebrates. New York: W.H. Freeman.<br />
Bullock, T. H. and Horridge, G. A. 1965b. Structure and function in the nervous<br />
system of invertebrates. New York: W.H. Freeman.<br />
Bulman, O. M. B. 1939. Muscle systems of some inarticulate brachiopods. Geol<br />
Mag 76:434-444.<br />
Carlson, S. J. 1995. Phylogenetic relationships among extant brachiopods.<br />
Cladistics 11:131-197.<br />
Carroll, S. 2005. From DNA to diversity: molecular genetics and the evolution of<br />
animal design. 2 ed. Oxford: Blackwell Publishing.<br />
Chernyshev, A. V. and Magarlamov, T. Y. 2010. The first data on the nervous<br />
system of hoplonemertean larvae (Nemertea, Hoplonemertea). Gen Biol<br />
430:48-50.<br />
Colomb, S., Joly, W., Bonneaud, N., and Maschat, F. 2008. A concerted action<br />
of engrailed and gooseberry-neuro in neuroblast 6-4 is triggering the<br />
formation of embryonic posterior commissure bundles. PLoS ONE<br />
3:e2197.<br />
Conway Morris, S. and Peel, J. S. 1995. Articulated halkieriids from the Lower<br />
Cambrian of North Greenland and their role in early protostome evolution.<br />
Philos Trans R Soc Lond B Biol Sci 347:305-358.<br />
Copf, T., Schröder, R., and Averof, M. 2004. Ancestral role of caudal genes in<br />
axis elongation and segmentation. Proc Natl Acad Sci USA 101:17711-<br />
17715.<br />
Cuvier, G. L. 1805. Leçons d’anatomie comparée de G. Cuvier. Vol. 3. Paris.<br />
Davidson, E. H. and Levine, M. S. 2008. Properties of developmental gene<br />
regulatory networks. Proc Natl Acad Sci USA 105:20063-20066.<br />
Davis, G. K., D’Alessio, J. A., and Patel, N. H. 2005. Pax3/7 genes reveal<br />
conservation and divergence in the arthropod segmentation hierarchy.<br />
Dev Biol 285:169-184.<br />
De Robertis, E. M. 2008. Evo-devo: variations on ancestral themes. Cell<br />
132:185-195.<br />
de Rosa, R., Grenier, J. K., Andreeva, T., Cook, C. E., Adoutte, A., Akam, M.,<br />
Carroll, S. B., and Balavoine, G. 1999. Hox genes in brachiopods and
30 References<br />
priapulids and protostome evolution. Nature 399:772-776.<br />
Duprey, P., Chowdhury, K., Dressler, G. R., Balling, R., Simon, D., Guenet, J.<br />
L., and Gruss, P. 1988. A mouse gene homologous to the Drosophila<br />
gene caudal is expressed in epithelial cells from the embryonic intestine.<br />
Genes Dev 2:1647-1654.<br />
Eernisse, D. J., Albert, J. S., and Anderson, F. E. 1992. Annelida and Arthropoda<br />
are not sister taxa: a phylogenetic analysis of spiralian metazoan<br />
morphology. Syst Biol 41:305-330.<br />
Field, K., Olsen, G., Lane, D., Giovannoni, S., Ghiselin, M., Raff, E., Pace, N.,<br />
and Raff, R. 1988. Molecular phylogeny of the animal kingdom. Science<br />
239:748-753.<br />
Freeman, G. 1993. Regional specification during embryogenesis in the articulate<br />
brachiopod Terebratalia. Dev Biol 160:196-213.<br />
Freeman, G. 2000. Regional specification during embryogenesis in the craniiform<br />
brachiopod Crania anomala. Dev Biol 227:219-238.<br />
Freeman, G. 2003. Regional specification during embryogenesis in<br />
Rhynchonelliform brachiopods. Dev Biol 261:268-287.<br />
Freeman, G. and Lundelius, J. W. 2005. The transition from planktotrophy to<br />
lecithotrophy in larvae of Lower Palaeozoic rhynchonelliform brachiopods.<br />
Lethaia 38:219-254.<br />
Fröbius, A. and Seaver, E. 2006. ParaHox gene expression in the polychaete<br />
annelid Capitella sp. I. Dev Genes Evol 216:81-88.<br />
Fuchs, J. and Wanninger, A. 2008. Reconstruction of the neuromuscular system<br />
of the swimming-type larva of Loxosomella atkinsae (Entoprocta) as<br />
inferred by fluorescence labelling and confocal microscopy. Org Divers<br />
Evol 8:325-335.<br />
Gabriel, W. and Goldstein, B. 2007. Segmental expression of Pax3/7 and<br />
Engrailed homologs in tardigrade development. Dev Genes Evol<br />
217:421-433.<br />
Giribet, G. 2008. Assembling the lophotrochozoan (=spiralian) tree of life. Philos<br />
Trans R Soc Lond B Biol Sci 363:1513-1522.<br />
Gruhl, A. 2009. Serotonergic and FMRFamidergic nervous systems in<br />
gymnolaemate bryozoan larvae. Zoomorphology 128:135-156.<br />
Gustus, R. M. and Cloney, R. A. 1972. Ultrastructural similarities between setae<br />
of brachiopods and polychaetes. Acta Zool 53:229-233.<br />
Halanych, K. M. 1995. Evidence from 18S ribosomal DNA that the lophophorates<br />
are protostome animals. Science 268:485-485.<br />
Halanych, K. M. 2004. The new view of animal phylogeny. Annu Rev Ecol Evol<br />
Syst 35:229-256.
References<br />
31<br />
Hancock, A. 1858. On the organization of the Brachiopoda. Phil. Trans. R. Soc.<br />
Lond. 148:791-869.<br />
Harlow, E. and Lane, D. 1999. Using antibodies: A laboratory manual. Cold<br />
Spring Harbor, New York: Cold Spring Harbor Laboratory Press.<br />
Harzsch, S. and Wanninger, A. 2010. Evolution of invertebrate nervous systems:<br />
the Chaetognatha as a case study. Acta Zool 91:35-43.<br />
Hatschek, B. 1888. Lehrbuch der Zoologie: eine morphologische Übersicht<br />
des Thierreiches zur Einführung in das Studium dieser Wissenschaft:<br />
G. Fischer.<br />
Hay-Schmidt, A. 1992. Ultrastructure and immunocytochemistry of the nervoussystem<br />
of the larvae of Lingula anatina and Glottidia sp. (Brachiopoda).<br />
Zoomorphology 112:189-205.<br />
Hay-Schmidt, A. 2000. The evolution of the serotonergic nervous system. Proc<br />
R Soc B 267:1071-1079.<br />
Hayashi, S., Drayton, B., Aurade, F., Rocancourt, D., Buckingham, M., and<br />
Relaix, F. 2010. Conserved functions of Pax3/7 during evolution. Dev<br />
Biol 344:528-529.<br />
Hejnol, A. and Martindale, M. Q. 2008. Acoel development indicates the<br />
independent evolution of the bilaterian mouth and anus. Nature 456:382-<br />
386.<br />
Hejnol, A., Obst, M., Stamatakis, A., Ott, M., Rouse, G. W., Edgecombe, G. D.,<br />
Martinez, P., Baguñà, J., Bailly, X., Jondelius, U., Wiens, M., Müller, W.<br />
E. G., Seaver, E., Wheeler, W. C., Martindale, M. Q., Giribet, G., and<br />
Dunn, C. W. 2009. Assessing the root of bilaterian animals with scalable<br />
phylogenomic methods. Proc R Soc B 276:4261-4270.<br />
Helmcke, J. G. 1939. Die Muskeln der Brachiopoden. Zool Jahrb Abt Syst Oekol<br />
Geogr Tiere 72:100-140.<br />
Holland, N. D. 2003. Early central nervous system evolution: an era of skin<br />
brains? Nat Rev Neurosci 4:617-627.<br />
Holland, P. W. H., Williams, N. A., and Lanfear, J. 1991. Cloning of segment<br />
polarity gene homologues from the unsegmented brachiopod<br />
Terebratulina retusa (Linnaeus). FEBS Letters 291:211-213.<br />
Huxley, T. H. 1853. On the morphology of the cephalous mollusca, as illustrated<br />
by the anatomy of certain Heteropoda and Pteropoda collected during<br />
the voyage of H.M.S. “Rattlesnake” in 1846-50. Phil. Trans. R. Soc.<br />
Lond. 143:29-65.<br />
Hyman, L. H. 1959. The lophophore coelomates - phylum Brachiopoda. In<br />
The Invertebrates Smaller Coelomate Groups, edited by L. H. Hyman.<br />
London: McGraw-Hill Book Company.
32 References<br />
James, M. A., Ansell, A. D., Collins, M. J., Curry, G. B., Peck, L. S., and Rhodes,<br />
M. C. 1992. Biology of living brachiopods. Adv Mar Biol 28:175-387.<br />
Jekely, G. and Arendt, D. 2007. Cellular resolution expression profiling using<br />
confocal detection of NBT/BCIP precipitate by reflection microscopy.<br />
Biotechniques 42:751-755.<br />
Kanzawa, N., Takano-Ohmuro, H., and Maruyama, K. 1995. Isolation and<br />
characterization of sea sponge myosin. Zool Sci 12:765-769.<br />
Kerner, P., Béhague, J., Simionato, E., Gouar, M. L., Balavoine, G., and Vervoort,<br />
M. 2009. Molecular analysis of the architecture and the development of<br />
the peripheral nervous system of the annelid Platynereis dumerilii. Mech<br />
Dev 126:S258-S258.<br />
Kowalevski, A. O. 1883. Observations sur le développement des brachiopodes<br />
(Analyse par Oehlert et Deniker). Arch Zool Exp Gen. 2:57-76.<br />
Lake, J. A. 1990. Origin of the Metazoa. Proc Natl Acad Sci USA 87:763-766.<br />
Lamarck, J. B. 1801. Classe premiere. La 5e du règne animal. Les Mollusques.<br />
In Système des Animaux sans vertèbres, edited by Déterville. Paris.<br />
Leys, S., Mackie, G., and Meech, R. 1999. Impulse conduction in a sponge. J<br />
Exp Biol 202:1139-1150.<br />
Linné, C. v. 1758. Systema naturae per regna tria naturae: secundum classes,<br />
ordines, genera, species, cum characteribus, differentiis, synonymis,<br />
locis. Vol. 1. Holmiae: Impensis Direct. Laurentii Salvii.<br />
Logan, A. 2007. Geographic distribution of extant articulated brachiopods. In<br />
Treatise on Invertebrate Paleontology, Part H, Brachiopoda, Revised,<br />
edited by P. A. Selden. Boulder, Colorado, and Lawrence, Kansas: The<br />
Geological Society of America, Inc. and The University of Kansas.<br />
Long, S. and Rebagliati, M. 2002. Sensitive two-color whole-mount in situ<br />
hybridizations using digoxygenin- and dinitrophenol-labeled RNA probes.<br />
Biotechniques 32:494-500.<br />
Lowe, C. J., Terasaki, M., Wu, M., Freeman, R. M., Jr., Runft, L., Kwan, K.,<br />
Haigo, S., Aronowicz, J., Lander, E., Gruber, C., Smith, M., Kirschner,<br />
M., and Gerhart, J. 2006. Dorsoventral patterning in hemichordates:<br />
insights into early chordate evolution. PLoS Biol 4:e291.<br />
Lowe, C. J., Wu, M., Salic, A., Evans, L., Lander, E., Stange-Thomann, N.,<br />
Gruber, C. E., Gerhart, J., and Kirschner, M. 2003. Anteroposterior<br />
patterning in hemichordates and the origins of the chordate nervous<br />
system. Cell 113:853-865.<br />
Lüter, C. 2000a. The origin of the coelom in Brachiopoda and its phylogenetic<br />
significance. Zoomorphology 120:15-28.<br />
Lüter, C. 2000b. Ultrastructure of larval and adult setae of Brachiopoda. Zool
References<br />
33<br />
Anz 239:75-90.<br />
Macdonald, P. M. and Struhl, G. 1986. A molecular gradient in early Drosophila<br />
embryos and its role in specifying the body pattern. Nature 324:537-<br />
545.<br />
Martindale, M. Q., Pang, K., and Finnerty, J. R. 2004. Investigating the origins<br />
of triploblasty: ‘mesodermal’ gene expression in a diploblastic animal,<br />
the sea anemone Nematostella vectensis (phylum, Cnidaria; class,<br />
Anthozoa). Development 131:2463-2474.<br />
Martinelli, C. and Spring, J. 2004. Expression pattern of the homeobox gene<br />
Not in the basal metazoan Trichoplax adhaerens. Gene Expr Patterns.<br />
4:443-447.<br />
McDougall, C., Chen, W.-C., Shimeld, S., and Ferrier, D. 2006. The development<br />
of the larval nervous system, musculature and ciliary bands of<br />
Pomatoceros lamarckii (Annelida): heterochrony in polychaetes. Front<br />
Zool 3:16.<br />
Meier, S. 1984. Somite formation and its relationship to metameric patterning of<br />
the mesoderm. Cell Differ 14:235-243.<br />
Morse, E. 1871. The Brachiopoda, a division of Annelida. In Proceedings of the<br />
American Association for Advances in Science, 19th meeting 1870.<br />
Morse, E. S. 1873. On the systematic position of the Brachiopoda. Boston:<br />
Press of A. A. Kingman.<br />
Nielsen, C. 1991. The development of the brachiopod Crania (Neocrania)<br />
anomala (O. F. Müller) and its phylogenetic significance. Acta Zool 72:7-<br />
28.<br />
Nielsen, C. 1995. Animal evolution: interrelationships of the living phyla. Oxford:<br />
Oxford University Press.<br />
Nielsen, C. and Worsaae, K. 2010. Structure and occurrence of cyphonautes<br />
larvae (Bryozoa, Ectoprocta). J Morphol 271:1094-1109.<br />
Noll, M. 1993. Evolution and role of Pax genes. Curr Opin Genet Dev 3:595-<br />
605.<br />
Orrhage, L. 1973. Light and electron microscope studies of some brachiopod<br />
and pogonophoran setae. Z. Morph. Tiere 74:253-270.<br />
Pires, A. and Woollacott, R. M. 1997. Serotonin and dopamine have opposite<br />
effects on phototaxis in larvae of the bryozoan Bugula neritina. Biol Bull<br />
192:399-409.<br />
Reed, C. G. and Cloney, R. A. 1977. Brachiopod tentacles - ultrastructure and<br />
functional significance of connective-tissue and myoepithelial cells in<br />
Terebratalia. Cell Tissue Res 185:17-42.<br />
Reichert, H. 2009. Evolutionary conservation of mechanisms for neural
34 References<br />
regionalization, proliferation and interconnection in brain development.<br />
Biol Lett 5:112-116.<br />
Richardson, J. R. and Watson, J. E. 1975. Locomotory adaptations in a freelying<br />
brachiopod. Science 189:381-382.<br />
Rieger, V., Perez, Y., Müller, C. H. G., Lipke, E., Sombke, A., Hansson, B. S., and<br />
Harzsch, S. 2010. Immunohistochemical analysis and 3D reconstruction<br />
of the cephalic nervous system in Chaetognatha: insights into the<br />
evolution of an early bilaterian brain? Invertebr Biol 129:77-104.<br />
Rowell, A. J. 1960. Some early stages in the development of the brachiopod<br />
Crania anomala (Müller). Ann. Mag. nat. Hist. 13:35-56.<br />
Rudwick, M. J. S. 1961. `Quick’ and `Catch’ adductor muscles in brachiopods.<br />
Nature 191:1021-1021.<br />
Rudwick, M. J. S. 1970. Living and fossil brachiopods. London: Hutchinson.<br />
Santagata, S. 2002. Structure and metamorphic remodeling of the larval<br />
nervous system and musculature of Phoronis pallida (Phoronida). Evol<br />
Dev 4:28-42.<br />
Santagata, S. and Zimmer, R. L. 2002. Comparison of the neuromuscular<br />
systems among actinotroch larvae: systematic and evolutionary<br />
implications. Evol Dev 4:43-54.<br />
Schmidt-Rhaesa, A. 2007a. Musculature. In The evolution of organ systems.<br />
Oxford: Oxford University Press.<br />
Schmidt-Rhaesa, A. 2007b. Nervous system. In The evolution of organ systems.<br />
Oxford: Oxford University Press.<br />
Scholtz, G. and Dohle, W. 1996. Cell lineage and cell fate in crustacean embryos<br />
- A comparative approach. Int J Dev Biol 40:211-220.<br />
Schram, F. R. 1991. Cladistic analysis of metazoan phyla and the placement of<br />
fossil Problematica. In The early evolution of Metazoa and the significance<br />
of problematic taxa, edited by A. M. Simonetta and S. Conway Morris:<br />
Cambridge University Press.<br />
Seaver, E. C., Thamm, K., and Hill, S. D. 2005. Growth patterns during<br />
segmentation in the two polychaete annelids, Capitella sp. I and<br />
Hydroides elegans: comparisons at distinct life history stages. Evol Dev<br />
7:312-326.<br />
Shimizu, K., Hunter, E., and Fusetani, N. 2000. Localisation of biogenic amines<br />
in larvae of Bugula neritina (Bryozoa: Cheilostomatida) and their effects<br />
on settlement. Mar Biol 136:1-9.<br />
Sommer, R. J. 2009. The future of evo-devo: model systems and evolutionary<br />
theory. Nat Rev Genet 10:416-422.<br />
Stricker, S. A. and Reed, C. G. 1985a. The ontogeny of shell secretion in
References<br />
35<br />
Terebratalia transversa (Brachiopoda, Articulata). 1. Development of the<br />
mantle. J Morphol 183:233-250.<br />
Stricker, S. A. and Reed, C. G. 1985b. The ontogeny of shell secretion in<br />
Terebratalia transversa (Brachiopoda, Articulata). 2. formation of the<br />
protegulum and juvenile shell. J Morphol 183:251-271.<br />
Telford, M. J. 2007. A single origin of the central nervous system? Cell 129:237-<br />
239.<br />
Thiemann, M. and Ruthmann, A. 1989. Microfilaments and microtubules in<br />
isolated fiber cells of Trichoplax adhaerens (Placozoa). Zoomorphology<br />
109:89-96.<br />
Trinh, L. A., McCutchen, M. D., Bonner-Fraser, M., Fraser, S. E., Bumm, L. A.,<br />
and McCauley, D. W. 2007. Fluorescent in situ hybridization employing<br />
the conventional NBT/BCIP chromogenic stain. Biotechniques 42:756-<br />
759.<br />
van Bemmelen, J. F. 1883. Untersuchungen über den anatomischen und<br />
histologischen Bau der Brachiopoda Testicardinia. Jen Zeit Nat 16:88-<br />
161.<br />
Venuti, J. M., Pepicelli, C., and Flowers, V. L. 2004. Analysis of sea urchin<br />
embryo gene expression by immunocytochemistry. Methods Cell Biol<br />
74:333-369.<br />
Voronezhskaya, E. E., Tsitrin, E. B., and Nezlin, L. P. 2003. Neuronal<br />
development in larval polychaete Phyllodoce maculata (Phyllodocidae).<br />
J Comp Neurol. 455:299-309.<br />
Wanninger, A. 2008. Comparative lophotrochozoan neurogenesis and larval<br />
neuroanatomy: Recent advances from previously neglected taxa. Acta<br />
Biol Hung 59:(Suppl.):127-136.<br />
Wanninger, A. 2009. Shaping the things to come: ontogeny of lophotrochozoan<br />
neuromuscular systems and the Tetraneuralia concept. Biol. Bull.<br />
216:293-306.<br />
Wanninger, A., Fuchs, J., and Haszprunar, G. 2007. Anatomy of the serotonergic<br />
nervous system of an entoproct creeping-type larva and its phylogenetic<br />
implications. Invertebr Biol 126:268-278.<br />
Watanabe, H., Fujisawa, T., and Holstein, T. W. 2009. Cnidarians and the<br />
evolutionary origin of the nervous system. Dev Growth Differ 51:167-<br />
183.<br />
Westfall, J. 1996. Ultrastructure of synapses in the first-evolved nervous<br />
systems. J Neurocytol 25:735-746.<br />
Westfall, J. A. and Elliott, C. F. 2002. Ultrastructure of the tentacle nerve plexus<br />
and putative neural pathways in sea anemones. Invertebr Biol 121:202-
36 References<br />
211.<br />
Williams, A., Carlson, S. J., Brunton, C. H. C., Holmer, L. E., and Popov, L.<br />
1996. A supra-ordinal classification of the Brachiopoda. Proc R Soc B<br />
351:1171-1193.<br />
Williams, A., James, M. A., Emig, C. C., Mackay, S., and Rhodes, M. C. 1997.<br />
Anatomy. In Treatise on Invertebrate Paleontology, Part H, Brachiopoda,<br />
Revised, edited by R. L. Kaesler. Lawrence, Kansas: Geological Society<br />
of America Inc. and The University of Kansas.<br />
Yatsu, N. 1902. On the development of Lingula anatina. J Coll Sci Tokyo 17:1-<br />
112.<br />
Younossi-Hartenstein, A., Green, P., Liaw, G.-J., Rudolph, K., Lengyel, J., and<br />
Hartenstein, V. 1997. Control of early neurogenesis of the Drosophila<br />
brain by the head gap genes tll, otd, ems,and btd. Dev Biol 182:270-<br />
283
Chapter II<br />
37<br />
Chapter II<br />
Altenburger, A. & Wanninger, A. 2009 Comparative larval<br />
myogenesis and adult myoanatomy of the rhynchonelliform<br />
(articulate) brachiopods Argyrotheca cordata, A. cistellula, and<br />
Terebratalia transversa. Frontiers in Zoology 6: 1-14
38 Chapter II<br />
Frontiers in Zoology<br />
BioMed Central<br />
Research<br />
Comparative larval myogenesis and adult myoanatomy of the<br />
rhynchonelliform (articulate) brachiopods Argyrotheca cordata, A.<br />
cistellula, and Terebratalia transversa<br />
Andreas Altenburger and Andreas Wanninger*<br />
Open Access<br />
Address: University of Copenhagen, Department of Biology, Research Group for Comparative Zoology, Universitetsparken 15, DK-2100<br />
Copenhagen Ø, Denmark<br />
Email: Andreas Altenburger - aaltenburger@bio.ku.dk; Andreas Wanninger* - awanninger@bio.ku.dk<br />
* Corresponding author<br />
Published: 3 February 2009<br />
Frontiers in Zoology 2009, 6:3<br />
doi:10.1186/1742-9994-6-3<br />
This article is available from: http://www.frontiersinzoology.com/content/6/1/3<br />
Received: 5 November 2008<br />
Accepted: 3 February 2009<br />
© 2009 Altenburger and Wanninger; licensee BioMed Central Ltd.<br />
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),<br />
which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.<br />
Abstract<br />
Background: Despite significant methodological progress, Brachiopoda remains one of the<br />
lophotrochozoan phyla for which no recent ontogenetic data employing modern methodologies<br />
such as fluorescence labelling and confocal microscopy are available. This is particularly astonishing<br />
given the ongoing controversy concerning its phylogenetic position. In order to contribute new<br />
morphogenetic data for phylogenetic and evolutionary inferences, we describe herein the ontogeny<br />
and myoanatomy of larvae and adults of the rhynchonelliform brachiopods Argyrotheca cordata, A.<br />
cistellula, and Terebratalia transversa using fluorescence F-actin labelling combined with confocal<br />
laserscanning microscopy.<br />
Results: Fully grown larvae of A. cordata and T. transversa consist of three distinct body regions,<br />
namely an apical lobe, a mantle lobe with four bundles of setae, and a pedicle lobe. Myogenesis is<br />
very similar in these two species. The first anlagen of the musculature develop in the pedicle lobe,<br />
followed by setae muscles and the mantle lobe musculature. Late-stage larvae show a network of<br />
strong pedicle muscles, central mantle muscles, longitudinal muscles running from the mantle to<br />
the pedicle lobe, setae pouch muscles, setae muscles, a U-shaped muscle, serial mantle muscles,<br />
and apical longitudinal as well as apical transversal muscles. Fully developed A. cistellula larvae differ<br />
from the former species in that they have only two visible body lobes and lack setae. Nevertheless,<br />
we found corresponding muscle systems to all muscles present in the former two species, except<br />
for the musculature associated with the setae, in larvae of A. cistellula. With our survey of the adult<br />
myoanatomy of A. cordata and A. cistellula and the juvenile muscular architecture of T. transversa we<br />
confirm the presence of adductors, diductors, dorsal and ventral pedicle adjustors, mantle margin<br />
muscles, a distinct musculature of the intestine, and striated muscle fibres in the tentacles for all<br />
three species.<br />
Conclusion: Our data indicate that larvae of rhynchonelliform brachiopods share a common<br />
muscular bodyplan and are thus derived from a common ancestral larval type. Comparison of the<br />
muscular phenotype of rhynchonelliform larvae to that of the other two lophophorate phyla,<br />
Phoronida and Ectoprocta, does not indicate homology of individual larval muscles. This may be<br />
due to an early evolutionary split of the ontogenetic pathways of Brachiopoda, Phoronida, and<br />
Ectoprocta that gave rise to the morphological diversity of these phyla.<br />
Page 1 of 14<br />
(page number not for citation purposes)
Chapter II<br />
39<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
Background<br />
Brachiopoda is a small lophophorate phylum with a<br />
prominent fossil record since the Lower Cambrium [1].<br />
More than 12.000 fossil and approximately 380 recent<br />
species are known to date [2,3]. The phylum is commonly<br />
divided into three taxa, the articulate Rhynchonelliformea<br />
and the two inarticulate clades Craniiformea and Linguliformea<br />
[4], and has traditionally been grouped together<br />
with Phoronida and Ectoprocta into the superphylum<br />
Lophophorata. However, this classification has recently<br />
been challenged by paleontological and molecular datasets.<br />
While some analyses employing morphological data<br />
assign Brachiopoda to Deuterostomia [e.g., [5,6]], recent<br />
molecular data either propose sistergroup relationships to<br />
various spiralian phyla including Mollusca, Annelida, and<br />
Nemertea [7-11], or support the notion that Phoronida<br />
are an ingroup of Brachiopoda [12,13].<br />
Apart from some mainly gross morphological studies [14-<br />
21], detailed data using modern techniques such as fluorescence<br />
labelling and confocal laserscanning microscopy<br />
are not yet available. This is especially true with respect to<br />
the development of the musculature, despite the fact that<br />
myo-anatomical features may provide useful characters<br />
for reconstructing phylogenetic relationships [22,23].<br />
Recently, some data on larval muscle development for the<br />
proposed brachiopod sister groups Phoronida and Ectoprocta<br />
have become available [24-28]. Accordingly, larval<br />
myogenesis in Brachiopoda constitutes an important gap<br />
of knowledge in comparative developmental studies on<br />
Lophophorata. With the first thorough, comparative<br />
account of brachiopod larval myogenesis provided herein<br />
for the rhynchonelliform species Argyrotheca cordata<br />
(Risso, 1826), Argyrotheca cistellula (Searles-Wood, 1841),<br />
and Terebratalia transversa (Sowerby, 1846), we aim at<br />
stimulating the discussion concerning lophophorate bodyplan<br />
evolution, phylogeny, and development. Furthermore,<br />
we contribute to questions concerning the<br />
muscular ground pattern of rhynchonelliform brachiopod<br />
larvae. We supplement our ontogenetic data with a<br />
detailed description of the adult muscle systems of all<br />
three species.<br />
Results<br />
Embryonic and larval development of Argyrotheca<br />
cordata<br />
Embryos and larvae of Argyrotheca cordata are brooded by<br />
the mother animal and are released as late-stage larvae<br />
competent to undergo metamorphosis. Accordingly, larval<br />
development is entirely lecithotrophic. After cleavage<br />
and gastrulation (Fig. 1A), a three-lobed larva is established,<br />
which comprises an anterior apical lobe, a mantle<br />
lobe in the mid-body region, and a posterior pedicle lobe<br />
(Fig. 1B–F). In very early three-lobed stages, the blast-<br />
Scanning development Figure 1electron of Argyrotheca micrographs cordata of the embryonic and larval<br />
Scanning electron micrographs of the embryonic and<br />
larval development of Argyrotheca cordata. Anterior<br />
faces upward and scale bars equal 50 μm. (A) Early gastrula<br />
with blastopore (arrow). (B) Ventral view of an embryo at<br />
the onset of differentiation of the three-lobed larval bodyplan<br />
comprising apical lobe (AL), mantle lobe (ML), and pedicle<br />
lobe (PL). The arrowhead points to the region of the larval<br />
apical ciliary tuft. The arrow points to the larval mouth which<br />
corresponds to the blastopore. (C) Dorsal view of a larva<br />
with distinct anlagen of the three body lobes. (D) Ventral<br />
view of a specimen of the same ontogenetic stage as the one<br />
in C with reduced larval apical ciliary tuft (arrowhead) and<br />
with the almost closed blastopore (arrow). (E) Three-lobed<br />
larva at the onset of setae formation (double arrowheads),<br />
dorso-lateral view. (F) Lateral view of a fully differentiated<br />
larva showing two of the four pairs of larval setae (double<br />
arrowheads) and a distinct primordial hump (asterisk).<br />
Page 2 of 14<br />
(page number not for citation purposes)
40 Chapter II<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
opore is visible at the base of the apical lobe (Fig. 1B). This<br />
larval mouth closes during subsequent larval development<br />
(Fig. 1D).<br />
The apical lobe is ciliated and bears, in early three lobed<br />
stages, an apical tuft which is lost in later stages (Fig. 1B,<br />
D). When the three lobes are fully established, four bundles<br />
of larval setae are formed at the posterior margin of<br />
the mantle lobe (Fig. 1E). Finally, in larvae competent to<br />
undergo metamorphosis, the anlage of the pedicle<br />
becomes visible as a distinct primordial hump at the posterior<br />
pole of the pedicle lobe (Fig. 1F).<br />
Myogenesis and adult myoanatomy of Argyrotheca<br />
cordata<br />
The larvae investigated were about 230–270 μm long and<br />
210–240 μm wide. The first F-actin-positive signal is visible<br />
as distinct spots in the area that later forms the mantle<br />
lobe (Fig. 2A). These distinct spots are F-actin-positive<br />
microvilli which are situated in the lower part of the setal<br />
sacs where the setae are formed [cf. [29]]. The strong fluorescence<br />
signal of the microvilli disappears once setae formation<br />
is completed, due to the increasing predominance<br />
of the larval musculature (Fig. 2D–F).<br />
The pedicle muscles start to form in three-lobed larvae<br />
that still lack setae (Fig. 2B). In older larvae with short<br />
setae (corresponding to the stage shown in Fig. 1E), setae<br />
muscles start to develop. These run from the setal pouches<br />
in anterior direction and connect to the apical longitudinal<br />
muscles at the border between apical and mantle lobe<br />
(lateral setae muscles) or to the central mantle muscles<br />
(dorsal setae muscles), respectively (Fig. 2C). The apical<br />
longitudinal muscles extend laterally within the apical<br />
lobe and terminate anteriorly at an apical transversal muscle<br />
(Fig. 2C). At this stage, longitudinal muscles are also<br />
found within the pedicle lobe. From there, they run into<br />
the mantle lobe, where they connect to longitudinal muscles<br />
which originate at the muscle interconnection point<br />
at the border between apical and mantle lobe. The larval<br />
gut rudiment is visible as a tube in the centre of the larvae<br />
(Fig. 2C).<br />
In fully developed larvae, setae pouch muscles are established<br />
and interconnected by a circular mantle muscle<br />
(Fig. 2D). From this circular mantle muscle emerge serial<br />
mantle muscles, which are dorsolaterally closed by the<br />
central mantle muscles. The central mantle muscles are<br />
connected to the dorsal setae muscles and to the apical<br />
longitudinal muscles at the border of the apical and the<br />
mantle lobe (Fig. 2E–F). Anteroventrally, the serial mantle<br />
muscles are enclosed by a U-shaped muscle which extends<br />
ventrally from the pedicle muscles towards the circular<br />
mantle muscle (Fig. 2D–F; see also additional file 1). The<br />
primordial hump is devoid of any musculature (Fig. 2E–<br />
F).<br />
Adult A. cordata studied were 0.8–1.3 mm wide and 0.9–<br />
1.4 mm long. We can confirm four pairs of muscles which<br />
have been described previously [30]. These are one pair of<br />
adductors and one pair of diductors, which attach to both<br />
the dorsal and to the ventral valve. In addition, there are<br />
two pairs of pedicle adjustors, one of which being<br />
attached to the ventral valve and the pedicle, and one<br />
being attached to the dorsal valve and the pedicle (Fig.<br />
3A–B). In addition, we found a distinct musculature in the<br />
tentacles of the lophophore and in the digestive system.<br />
Each tentacle contains several bands of striated muscle<br />
fibers (Fig. 3D–E), while the stomach and intestine are<br />
each lined by numerous delicate ring muscles (Fig. 3C).<br />
Moreover, minute muscles are distributed along the dorsal<br />
and ventral mantle margin, which probably function<br />
as mantle retractor muscles. These mantle retractors are<br />
abundant and are oriented perpendicularly to the mantle<br />
margin that lines the shell (Fig. 3A–B).<br />
Myogenesis and adult myoanatomy of Argyrotheca<br />
cistellula<br />
Similar to Argyrotheca cordata, larvae of A. cistellula are lecithotrophic<br />
and are brooded by the mother animal. A. cistellula<br />
larvae lack setae and the mantle lobe encloses the<br />
pedicle lobe during development. Thus, the fully developed<br />
larvae have only two visible lobes, namely the apical<br />
and the mantle lobe. The investigated larvae were around<br />
117–139 μm long and 78–104 μm wide. The first muscles<br />
appear in larvae with all lobes fully differentiated. These<br />
are two dorsal mantle muscles which extend dorsally from<br />
anterior to posterior in the mantle lobe (Fig. 4A). Parallel<br />
and further lateral to these dorsal mantle muscles run the<br />
early lateral mantle muscles, and the first rudiments of the<br />
serial mantle muscles arise at this stage in the mantle lobe.<br />
These develop subsequently into a network of muscles<br />
that extends dorsally and ventrally from the two lateral<br />
mantle muscles (Fig. 4A–F). These lateral mantle muscles<br />
connect to the apical longitudinal muscles at the anterior<br />
pole and to the posterior muscle ring at the posterior pole<br />
of the larvae (Fig 4B–F). During subsequent development,<br />
the ventral mantle muscles and the pedicle muscles<br />
emerge (Fig. 4C). The pedicle muscles, situated in the centre<br />
of the mantle lobe, are the most prominent muscles in<br />
fully grown larvae (Fig. 4D). They connect to the apical<br />
longitudinal muscles, which in turn are in contact with<br />
the apical transversal muscles. The latter form a muscle<br />
ring in the apical lobe (Fig. 4E–F). The musculature of<br />
fully developed larvae includes the pedicle muscles, which<br />
are connected to the apical longitudinal muscles, the ventral<br />
mantle muscles, and the dorsal mantle muscles that<br />
connect to the pedicle muscles. Furthermore, serial mantle<br />
muscles, which extend dorsally and ventrally from the<br />
lateral mantle muscles, are present. Ventrally, the serial<br />
mantle muscles terminate at the ventral mantle muscles<br />
(Fig. 4F).<br />
Page 3 of 14<br />
(page number not for citation purposes)
Chapter II<br />
41<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
Myogenesis in Argyrotheca cordata<br />
Figure 2<br />
Myogenesis in Argyrotheca cordata. CLSM maximum projection micrographs, anterior faces upward. F-actin is labelled in<br />
red and cell nuclei are labelled in blue to indicate the outline of the specimens. Scale bars equal 50 μm. (A) Early larva in dorsal<br />
view with the first F-actin signals from microvilli (mi) within the setal canals. (B) Early three-lobed larval stage, postero-dorsal<br />
view, showing apical lobe (AL), mantle lobe (ML), pedicle lobe (PL), first rudiments of the pedicle musculature (pm), and microvilli<br />
(mi) in the setae pouches. (C) Larval stage with fully differentiated lobes and short setae in ventral view (corresponding to<br />
the larval stage shown in Fig. 1E). Visible are the apical transversal muscle (atm), the apical longitudinal muscles (alm), the interconnecting<br />
apical muscles (iam), the interconnecting mantle muscles (imm), the longitudinal muscles (lm), the foregut rudiment<br />
(fg), the hindgut rudiment (hg), the pedicle muscles (pm), microvilli (mi), the setae pouch musculature (arrowheads), and the<br />
setae muscles (sm). (D) Lateral right view of a fully developed three-lobed larva with the U-shaped muscle (empty arrows) on<br />
the ventral side. At this stage, the setae pouches are interconnected by a circular mantle muscle (arrow). New at this stage are<br />
the central mantle muscles (empty arrowhead). Further indicated are the setae pouch musculature (arrowheads), the setae<br />
muscles (sm), the serial mantle muscles (double arrowheads), the pedicle musculature (pm), and the apical longitudinal muscles<br />
(alm). (E) Same stage as in D, ventro-lateral view. The U-shaped muscle (empty arrows) is directly connected to the pedicle<br />
muscles (pm). In addition, the apical transversal muscle (atm), the apical longitudinal muscles (alm), the serial mantle muscles<br />
(double arrowhead), the central mantle muscles (empty arrowheads), the setae pouch muscles (arrowheads), the setae muscles<br />
(sm), the circular mantle muscle (arrow), and the primordial hump (asterisk) are indicated. (F) Fully grown larva in ventral<br />
view with circular mantle muscle (arrows), serial mantle muscles (double arrowheads), setae pouch muscles (arrowheads),<br />
setae muscles (sm), pedicle muscles (pm), longitudinal muscles (lm), apical longitudinal muscles (alm), apical transversal muscle<br />
(atm), interconnecting apical muscles (iam), primordial hump (asterisk), and central mantle muscles (empty arrowheads).<br />
Page 4 of 14<br />
(page number not for citation purposes)
42 Chapter II<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
Similar to the condition found in Argyrotheca cordata, four<br />
pairs of shell muscles are found in adult A. cistellula (Fig.<br />
5). One pair of shell adductors attaches medially to the<br />
dorsal and to the ventral valve (Fig. 5). Two pairs of pedicle<br />
adjustors extend posterior into the pedicle, whereby<br />
one attaches to the dorsal and one to the ventral valve.<br />
Finally, one pair of diductors attaches at the posterior end<br />
of the ventral valve and runs to the dorsal valve.<br />
Each tentacle of the lophophore contains a number of striated<br />
muscle fibres. Mantle margin muscles are arranged<br />
perpendicularly to the shell periphery along the edge of<br />
the dorsal and the ventral valve (Fig. 5A–B).<br />
Myogenesis, metamorphosis, and juvenile myoanatomy of<br />
Terebratalia transversa<br />
Larvae of Terebratalia transversa are lecithotrophic and<br />
develop for approximately four days at 11°C in the water<br />
column until they are competent to undergo metamorphosis.<br />
The investigated larvae were three-lobed, 120–178<br />
μm long and 94–141 μm wide, whereby the pedicle lobe<br />
was partly overgrown by the mantle lobe. The first developing<br />
muscles are the pedicle muscles and early rudiments<br />
of the serial mantle muscles (Fig. 6A). Thereafter,<br />
the musculature of the four setae pouches forms (Fig. 6B).<br />
In later stages, the setae pouch muscles interconnect with<br />
the circular mantle muscle (Fig. 6C). A U-shaped muscle<br />
extends on the ventral side of the larvae from the pedicle<br />
muscles towards the circular mantle muscle. The serial<br />
mantle muscles and the setae muscles span between the<br />
circular mantle muscle and the U-shaped muscle strand.<br />
The latter run from the setae pouches to the central mantle<br />
muscles (Fig. 6D). The central mantle muscles extend<br />
from the dorsal setae muscles, which run from the dorsal<br />
setae pouches towards the apical lobe. They connect to the<br />
apical longitudinal muscles at the border of the apical and<br />
the mantle lobe (Fig. 6D). Subsequently, the apical musculature<br />
develops, which consists of an apical transversal<br />
muscle and two lateral apical longitudinal muscles that<br />
are connected to the serial mantle muscles (Fig. 6E). In<br />
late three-lobed larvae, the pedicle muscles are, together<br />
with the central mantle muscles, the most prominent<br />
muscular structures. The central mantle muscles connect<br />
to the serial mantle muscles, the setae pouch muscles, the<br />
setae muscles, and the apical musculature (Fig. 6F).<br />
During metamorphosis, parts of the larval musculature<br />
appear to get resorbed and juvenile muscles develop (Fig.<br />
7A). We were, however, unable to clarify whether or not<br />
certain components of the larval musculature are incorporated<br />
into the juvenile muscular bodyplan.<br />
The juvenile musculature comprises early rudiments of<br />
the tentacle muscles, early rudiments of the mantle margin<br />
musculature, the musculature of the intestine, adductors,<br />
ventral pedicle adjustors which are connected to the<br />
diductors, and dorsal pedicle adjustors (Fig. 7B–D).<br />
Discussion<br />
Comparison of larval and adult rhynchonelliform<br />
myoanatomy<br />
The gross morphology of Argyrotheca cistellula differs considerably<br />
from that of A. cordata and Terebratalia transversa<br />
in that the pedicle lobe gets enclosed by the mantle lobe<br />
during development [19]. Thus, A. cistellula appears twolobed<br />
and lacks setae, while the other two species express<br />
three distinct body lobes and setae. Despite these differences,<br />
myogenesis follows a similar pattern in all three<br />
species (Table 1). When fully developed, prominent pedicle<br />
muscles, apical longitudinal as well as apical transversal<br />
muscles, and serial mantle muscles are present in all<br />
three species. In addition, A. cordata and T. transversa<br />
show a circular mantle muscle which we consider homologous<br />
to the posterior muscle ring in A. cistellula. This<br />
homology is based on the similar position of this muscle<br />
in the mantle lobe and the fact that the U-shaped muscle<br />
of A. cordata and T. transversa and the ventral mantle muscles<br />
of A. cistellula all insert at this muscle. The central<br />
mantle muscles of A. cordata and T. transversa are in our<br />
opinion homologous to the dorsal mantle muscles of A.<br />
cistellula due to the similar position of these muscles and<br />
their connection to the apical and the serial mantle muscles<br />
in all three species. The U-shaped muscle of A. cordata<br />
and T. transversa corresponds to the ventral mantle muscles<br />
in A. cistellula due to their similar position and the fact<br />
that these muscles enclose the serial mantle muscles<br />
antero-ventrally.<br />
Despite these similarities, we found distinct differences in<br />
the myoanatomy of the three species investigated. As<br />
such, the setae pouch muscles, the setae muscles, and the<br />
longitudinal muscles, which run from the mantle lobe to<br />
the pedicle lobe, are only present in A. cordata and T. transversa,<br />
while the lateral mantle muscles are only present in<br />
larvae of A. cistellula. These differences between A. cistellula<br />
on the one hand and A. cordata and T. transversa on<br />
the other correspond to the gross morphological observation<br />
that A. cistellula lacks setae.<br />
Larval setae in brachiopods have been proposed to function<br />
as a defence device and to control buoyancy [31]. The<br />
setae of A. cistellula larvae have probably been secondarily<br />
lost, as these larvae are brooded and may settle shortly<br />
after release from the mother animal. However, A. cordata<br />
larvae have retained their setae despite being brooded,<br />
which may hint towards an extended planktonic period of<br />
these larvae.<br />
The muscles in the pedicle lobe have been proposed earlier<br />
to be of functional use during metamorphosis<br />
Page 5 of 14<br />
(page number not for citation purposes)
Chapter II<br />
43<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
Adult myoanatomy of Argyrotheca cordata<br />
Figure 3<br />
Adult myoanatomy of Argyrotheca cordata. F-actin is labelled in red and cell nuclei are labelled in blue. Scale bars equal<br />
100 μm in all aspects except in E, where it equals 25 μm. (A) Overlay of CLSM maximum projection micrograph and light<br />
micrograph, anterior faces upward, dorsal view. Indicated are the tentacle muscles (tm), the mantle margin muscles (mm), the<br />
tentacles of the lophophore (te), the mantle cavity (mc), the intestine (in), the shell (s), the adductors (ad), the ventral pedicle<br />
adjustors (vpa), which extend from the ventral valve into the pedicle, the dorsal pedicle adjustors (dpa), which extend from the<br />
dorsal valve into the pedicle, and the diductors (di). One diductor is lacking as a result of the removal of the animal from the<br />
substrate. (B) Overlay of a CLSM maximum projection micrograph and a light micrograph, anterior faces upward, ventral view.<br />
Indicated are the same structures as in A. (C) Enlarged view of the ring musculature lining the intestine (in). In addition, one<br />
adductor (ad), the diductors (di), and the ventral pedicle adjustors (vpa) are visible. (D) Enlarged view of the tentacles of the<br />
lophophore and the corresponding tentacle musculature (tm). (E) Detail of a tentacle muscle fibre showing typical striation pattern<br />
(double arrows).<br />
Page 6 of 14<br />
(page number not for citation purposes)
44 Chapter II<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
[32,33]. When larvae settle, a glandular region at the tip of<br />
the primordial hump functions as site of attachment to<br />
the substrate [34]. Subsequently, the primordial hump<br />
forms the first rudiment of the juvenile pedicle. After larval<br />
settlement, the mantle lobe is inverted over the apical<br />
lobe and eventually forms the juvenile mantle. The apical<br />
lobe gets enclosed by the valves and forms the lophophore<br />
and all anterior adult structures [32,35]. At the<br />
onset of metamorphosis, the U-shaped muscle may, due<br />
to its connection to the pedicle muscles and the circular<br />
mantle muscle, aid in inverting the mantle lobe. During<br />
metamorphosis, the larval pedicle muscles are still present<br />
at the time of ventral pedicle adjustor and diductor formation.<br />
However, whether the larval pedicle muscles are<br />
resorbed or are (partly) incorporated into the juvenile<br />
diductor and/or pedal adjustor muscles could not be clarified<br />
by the present study.<br />
Argyrotheca cordata is the sole species from this study for<br />
which data on the larval myoanatomy had previously<br />
been available. In the first descriptions from 1873 and<br />
1883, "muscles abdominaux", that run from the pedicle<br />
lobe into the mantle lobe, had been identified [14,30]. A<br />
different description was given slightly later, when a network<br />
of muscles in the fully developed larva was<br />
described. The muscles were denoted "Muskel des lateralen<br />
Borstenbündels", "Muskel des medialen Borstenbündels",<br />
"musculus contractor", "musculus rotator<br />
dorsalis", and "musculus abductor" [15]. Our findings<br />
confirm the results of the first papers with respect to the<br />
pedicle muscles and the setae muscles. However, in our<br />
specimens, the pedicle muscles were not directly connected<br />
to the setae muscles as depicted in the first descriptions,<br />
but were instead connected to the U-shaped muscle.<br />
In adult Argyrotheca cordata, four pairs of muscles had<br />
been identified previously [30]. The pair of adductor muscles<br />
has two insertion sites, one anterior to the other at the<br />
dorsal valve, and an additional one at the ventral valve.<br />
The pair of diductor muscles inserts at the posterior part<br />
of both the ventral and the dorsal valve. One of the two<br />
pairs of adjustors inserts at the ventral valve and the pedicle,<br />
while the other pair inserts at the dorsal valve and the<br />
pedicle [30].<br />
The muscular systems of adult A. cordata and A. cistellula<br />
are similar to each other and comprise one pair of adductors,<br />
two pairs of pedicle adjustors and one pair of diductors.<br />
The tentacles contain several fibres of striated<br />
musculature which have previously been described as<br />
"rows of striated fusiform myoepithelial cells" in the<br />
lophophore of T. transversa [36].<br />
For the juvenile musculature of Terebratalia transversa we<br />
followed the nomenclature used by Eshleman and<br />
Wilkens [37]. The juvenile musculature, five days after<br />
metamorphosis, comprises rudiments of the tentacle<br />
muscles, rudiments of the mantle margin musculature,<br />
one pair of adductors, one pair of diductors, one pair of<br />
dorsal, and one pair of ventral pedicle adjustors. The ventral<br />
pedicle adjustors are connected to the diductors in the<br />
juvenile.<br />
Comparative myogenesis of Lophophorata<br />
For the Phoronida, data on muscle development are currently<br />
available for three species, namely Phoronis pallida,<br />
P. harmeri, and P. architecta [24,26,27]. The larvae of these<br />
species are of the actinotroch-type and differ considerably<br />
from brachiopod larvae in both their gross anatomy and<br />
in their lifestyle, because these phoronid larvae are planktotrophic,<br />
while the brachiopod larvae investigated herein<br />
are of the typical three-lobed, lecithotrophic type. Accordingly,<br />
a considerable part of the larval phoronid musculature<br />
is linked to the digestive system (e.g., the oesophageal<br />
ring muscles) and to the maintenance of a cylindrical<br />
body shape (e.g., a meshwork of circular and longitudinal<br />
muscles in the bodywall). In addition, trunk retractor<br />
muscles, that originate from the posterior collar ring muscles<br />
and insert in the telotrochal region, are present in<br />
phoronid larvae [27]. The collar region contains mainly<br />
ring muscles and few longitudinal muscles. The subumbrellar<br />
and exumbrellar layers of the hood contain circular<br />
muscles and a series of longitudinal muscles, which, in<br />
the exumbrellar layer, function as hood elevators [27].<br />
Furthermore, the tentacles of phoronid actinotroch larvae<br />
contain elevator and depressor muscles which consist of<br />
two loops in the elevators and a single loop in the depressors.<br />
These tentacle muscles are interconnected by the ring<br />
muscle of the collar [27]. We did not identify any muscles<br />
in the larvae of the three brachiopod species described<br />
herein that could potentially correspond to the actinotroch<br />
muscle systems known so far.<br />
The muscular architecture in ectoproct larvae is very<br />
diverse, thus following the high plasticity of larval gross<br />
morphology and the notion that lecithotrophic larvae<br />
might have evolved up to six times within Ectoprocta [38].<br />
To date, the larval muscular systems have been described<br />
for Membranipora membranacea (cyphonautes larva), Flustrellidra<br />
hispida (pseudocyphonautes larva), Celleporaria<br />
sherryae and Schizoporella floridana (both coronate larva),<br />
Bowerbankia gracilis (vesiculariform larva), Bugula stolonium<br />
and B. fulva (both buguliform larva), Sundanella<br />
sibogae, Nolella stipata, Amathia vidovici, Aeverrillia setigera,<br />
and Alcyonidium gelatinosum (all ctenostome larva), and<br />
Crisia elongata (cyclostome larva) [25,28]. Recently, a<br />
number of homologies have been proposed for various<br />
larval ectoproct muscle systems [25]. These are the coronal<br />
ring muscle, which underlies the ciliated, ring-shaped<br />
swimming organ of most larval types, the anterior median<br />
Page 7 of 14<br />
(page number not for citation purposes)
Chapter II<br />
45<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
Myogenesis in Argyrotheca cistellula<br />
Figure 4<br />
Myogenesis in Argyrotheca cistellula. Overlay of CLSM maximum projection micrograph and light micrograph, anterior<br />
faces upward. Scale bars equal 50 μm. Note that only two lobes are visible: the apical lobe (AL) and the mantle lobe (ML),<br />
which encloses the pedicle lobe. (A) Early larva in dorsal view with the dorsal mantle muscles (empty arrowheads), the early<br />
lateral mantle muscles (lmm), and early rudiments of the serial mantle muscles (double arrowheads). (B) Dorsal view of a later<br />
larval stage with the lateral mantle muscle strand (lmm), rudiments of the posterior muscle ring (arrow), dorsal mantle muscles<br />
(empty arrowheads), and the serial mantle muscles (double arrowhead). (C) Later larva in ventro-lateral left view with pedicle<br />
muscles (pm) that are connected to the ventral mantle muscles (empty arrows). The serial mantle muscles (double arrowhead)<br />
are connected to the lateral mantle muscles (lmm), the apical longitudinal muscles (alm) start to develop, and the early posterior<br />
muscle ring is visible (arrow). (D) Same stage as in C, dorsal view. The pedicle muscles (pm) are prominent and connect to<br />
the dorsal mantle muscles (empty arrowheads). In addition, the lateral mantle muscles (lmm), the serial mantle muscles (double<br />
arrowheads), a part of the posterior muscle ring (arrow), and the apical longitudinal muscles (alm) are visible. (E) Fully developed<br />
larva, ventral view. The apical transversal (atm) and the apical longitudinal muscles (alm) are fully developed and connect<br />
to the pedicle muscles (pm). The connection between pedicle muscles and dorsal mantle muscles (empty arrowheads) is visible<br />
in the anterior region of the pedicle muscles. Further indicated are the ventral mantle muscles (empty arrows), the serial mantle<br />
muscles (double arrowheads), the lateral mantle muscles (lmm), and the area of the posterior muscle ring (arrow). (F) Same<br />
larval stage as in E, ventro-lateral left view. The pedicle muscles (pm) are the most prominent muscles in the centre of the mantle<br />
lobe. They are connected to the apical longitudinal muscles (alm), which terminate at the apical transversal muscle (atm),<br />
which in turn forms a muscle ring in the apical lobe. The ventral mantle muscles (empty arrows) and dorsal mantle muscles<br />
(empty arrowhead) are also connected to the pedicle muscles. The serial mantle muscles (double arrowhead) extend dorsally<br />
and ventrally from the lateral mantle muscles (lmm). The latter terminate at the posterior muscle ring (arrow).<br />
Page 8 of 14<br />
(page number not for citation purposes)
46 Chapter II<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
muscle, which runs anteriorly from ventral to dorsal in<br />
most species, lateral muscles, which project laterally in<br />
dorso-ventral direction in most larvae, longitudinal muscles<br />
along the posterior body axis, and transversal muscles,<br />
which are situated transversally in the central body<br />
region of F. hispida, M. membranacea, and A. gelatinosum.<br />
Besides these proposed homologous muscles, each larval<br />
type shows unique muscles in the body wall and/or inside<br />
the larval body, reflecting at least partly the functional<br />
adaptations to a planktotrophic versus a lecithotrophic<br />
lifestyle. No muscles corresponding to any of the ectoproct<br />
muscle types were found in the brachiopod species<br />
investigated in this study (and noticeably no homologous<br />
muscles between the lecithotrophic ectoproct and brachiopod<br />
larval types could be identified), again demonstrating<br />
the high plasticity of lophophorate larval anatomy.<br />
Conclusion<br />
All rhynchonelliform brachiopod larvae studied to date<br />
are three-lobed with four bundles of setae [39], except for<br />
the larva of Argyrotheca cistellula, which is externally<br />
bilobed and lacks setae, and the three-lobed thecideid larvae,<br />
which likewise lack setae [40]. Despite these gross<br />
morphological differences, myogenesis in the three brachiopod<br />
species investigated is very similar. Thus, we propose<br />
a larval muscular groundpattern for<br />
rhynchonelliform brachiopods comprising apical longitudinal<br />
muscles, apical transversal muscles, circular mantle<br />
muscles, central mantle muscles, longitudinal muscles,<br />
serial mantle muscles, pedicle muscles, setae pouch muscles,<br />
setae muscles, and a U-shaped muscle. However, a<br />
final statement can only be made once data on the musculature<br />
of theceid and rhynchonellid larvae become<br />
available.<br />
Comparing this proposed larval muscular groundpattern<br />
to the hitherto investigated phoronids, ectoprocts, and<br />
spiralian taxa such as polychaetes, molluscs,<br />
plathelminths or entoprocts does not reveal any homologies<br />
of larval brachiopod muscles and the muscles of other<br />
lophotrochozoan larvae, regardless of whether the respective<br />
larvae are lecithotrophic or planktotrophic [23,41-<br />
47]. From these data we conclude that the ontogenetic<br />
pathways of the individual lophophorate phyla have split<br />
early in evolution from that of other Lophotrochozoa,<br />
which then resulted in the wide morphological diversity<br />
of larval and adult lophophorate bodyplans.<br />
Methods<br />
Animal collection and fixation<br />
Argyrotheca cordata and A. cistellula<br />
Adults were obtained from encrusting coralline red algae<br />
(coralligène), which was collected in the vicinity of the<br />
Observatoire Océanologique de Banyuls-sur-mer, France<br />
(42°29'27.51" N; 3°08'07.67" E), by SCUBA from 30–40<br />
m depth in July 2002 and June 2007. All developmental<br />
stages from unfertilized eggs to fully differentiated larvae<br />
were obtained by dissection from the adults. The specimens<br />
were relaxed at room temperature in 7.14% MgCl 2 ,<br />
fixed in 4% paraformaldehyde (PFA) in 0.1 M phosphate<br />
Adult myoanatomy of Argyrotheca cistellula<br />
Figure 5<br />
Adult myoanatomy of Argyrotheca cistellula. Overlay of CLSM maximum projection micrograph and light micrograph,<br />
anterior faces upward. Scale bars equal 300 μm. (A) Dorsal view. (B) Ventral view. Indicated are the mantle margin muscles<br />
(mm), the shell (s), the adductors (ad), the diductors (di), the dorsal pedicle adjustor (dpa), the ventral pedicle adjustor (vpa),<br />
the intestine (in), the mantle cavity (mc), and the tentacle muscles (tm).<br />
Page 9 of 14<br />
(page number not for citation purposes)
Chapter II<br />
47<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
Myogenesis in Terebratalia transversa<br />
Figure 6<br />
Myogenesis in Terebratalia transversa. Overlay of CLSM maximum projection micrograph and light micrograph, anterior<br />
faces upward. Scale bars equal 50 μm. (A) Ventral view of an early three-lobed stage with apical lobe (AL), mantle lobe (ML),<br />
and pedicle lobe (PL). Discernable are the pedicle musculature (pm), the first anlagen of the serial mantle muscles (double<br />
arrowhead), and the setae (se). (B) Ventral view of a slightly older larva with prominent pedicle musculature (pm), anlagen of<br />
the setae pouch musculature (arrowheads), and setae (se). (C) Later larval stage, ventral view with pedicle musculature (pm),<br />
setae pouch muscles (arrowhead), serial mantle muscles (double arrowhead), and central mantle muscles (empty arrowheads),<br />
which are extensions of the dorsal setae muscles. The serial mantle muscles are posteriorly connected to the circular mantle<br />
muscle (arrows) and antero-ventrally connected to the U-shaped muscle (empty arrows), which extends from the pedicle muscles<br />
to the circular mantle muscle. (D) Lateral view of a later larva with the muscle systems described in C. In addition, the first<br />
anlagen of the apical longitudinal musculature (alm), the setae muscles (sm), and the setae (se) are visible. (E) Same stage as in<br />
D with prominent pedicle muscles (pm) that are connected to the apical longitudinal muscles (alm). The latter connect to the<br />
apical transversal muscle (atm). In addition, the setae pouch muscles (arrowheads), the setae muscles (sm), and the setae (se)<br />
are indicated. (F) Fully developed larva, ventral view, with central mantle muscles (empty arrowheads), pedicle muscles (pm),<br />
circular mantle muscle (arrows), U-shaped muscle (empty arrows), serial mantle muscles (double arrowheads), setae pouch<br />
musculature (arrowheads), setae muscles (sm), apical longitudinal muscles (alm), apical transversal muscle (atm), and setae (se).<br />
Page 10 of 14<br />
(page number not for citation purposes)
48 Chapter II<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
Metamorphosis and adult myoanatomy of Terebratalia transversa<br />
Figure 7<br />
Metamorphosis and adult myoanatomy of Terebratalia transversa. (A-C) Overlay of CLSM maximum projection<br />
micrograph and light micrograph, anterior faces upward. F-actin is labelled in red and cell nuclei are labelled in blue. Scale bars<br />
equal 50 μm. (A) Larva during metamorphosis. A mosaic of larval and juvenile features are present including the pedicle (pe),<br />
the larval pedicle muscles (pm), the first rudiments of the juvenile tentacle musculature (tm), one diductor (di), and the ventral<br />
pedicle adjustors (vpa). (B) Juvenile 5 days after metamorphosis, dorsal view with the remaining larval setae (se), the mantle<br />
margin muscles (mm), the tentacle muscles (tm), the adductors (ad), the musculature of the intestine (in), the diductors (di),<br />
the ventral pedicle adjustors (vpa), the dorsal pedicle adjustors (dpa), and the pedicle (pe). (C) Juvenile 5 days after metamorphosis,<br />
ventral view with the remaining larval setae (se), rudiments of the mantle margin muscles (mm), rudiments of the tentacle<br />
muscles (tm), the adductors (ad), the ventral pedicle adjustors (vpa), the diductors (di), the dorsal pedicle adjustors (dpa),<br />
and the pedicle (pe). (D) Reconstruction of the 3D arrangement of the juvenile musculature based on the CLSM dataset used<br />
in C showing the dorsal pedicle adjustors (red), the adductors (dark blue), the mantle margin muscles (light blue), and the tentacle<br />
muscles (magenta). The ventral pedicle adjustors (yellow) are ventrally connected to the diductors (green).<br />
Page 11 of 14<br />
(page number not for citation purposes)
Chapter II<br />
49<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
buffer (PB) for 2 hours or for 3–5 hours, and subsequently<br />
washed thrice with 0.1 M PB for 15 min each. The samples<br />
were stored in 0.1 M PB with 0.1% NaN 3 at 4°C. Material<br />
fixed for 2 hours was used for immunocytochemistry<br />
(ICC) and material fixed for 3–5 hours was used for scanning<br />
electron microscopy (SEM).<br />
Terebratalia transversa<br />
Adults were collected in the San Juan Archipelago, USA, in<br />
the vicinity of the Friday Harbor Laboratories, and were<br />
kept in running seawater tables. To obtain larvae, females<br />
were dissected and their eggs transferred into beaker<br />
glasses with filtered seawater. The seawater was changed<br />
several times in order to wash off follicle cells, and the<br />
eggs were left overnight for germinal vesicle breakdown.<br />
Males were opened and left in filtered seawater overnight.<br />
Thereafter, their testes were scraped out, macerated, and<br />
diluted with filtered seawater to obtain a sperm suspension.<br />
Prior to fertilization, sperm cells were activated by<br />
adding three drops of a 1 M Tris buffer solution (Sigma-<br />
Aldrich, St. Louis, MO, USA) to approximately 50 ml of<br />
sperm suspension. Larvae were maintained in embryo<br />
dishes at around 11°C and the filtered seawater was<br />
changed twice daily. Free swimming larvae, metamorphic<br />
stages, and juveniles five days after metamorphosis were<br />
relaxed in 7.14% MgCl 2 and fixed in 4% PFA in 0.1 M PB<br />
for 30 min at room temperature. Larvae were washed<br />
thrice for 15 min in 0.1 M PB and stored in 0.1 M PB with<br />
0.1% NaN 3 at 4°C.<br />
Scanning electron microscopy<br />
For scanning electron microscopy (SEM), the specimens<br />
were postfixed in 1% OsO 4 , dehydrated in a graded acetone<br />
series, critical point dried, and sputter coated with<br />
gold. Digital images were acquired using a LEO 1430 VP<br />
SEM (Zeiss, Jena, Germany).<br />
Table 1: Comparative larval myoanatomy of the rhynchonelliform brachiopods Argyrotheca cordata, Terebratalia transversa, and A.<br />
cistellula<br />
Species<br />
Muscle Argyrotheca cordata Terebratalia<br />
transversa<br />
Argyrotheca<br />
cistellula<br />
Location<br />
Symbol in figures<br />
apical longitudinal<br />
muscles<br />
apical transversal<br />
muscle<br />
+ + + apical lobe alm<br />
+ + + (apical muscle ring) apical lobe atm<br />
central mantle muscles + + +<br />
(dorsal mantle<br />
muscles)<br />
mantle lobe<br />
empty arrowheads<br />
circular mantle muscle + + +<br />
(posterior muscle ring)<br />
mantle lobe<br />
arrows<br />
lateral mantle muscle - - + mantle lobe lmm<br />
longitudinal muscles + + - mantle and pedicle<br />
lobe<br />
lm<br />
pedicle muscles + + + pedicle lobe pm<br />
serial mantle muscles + + + mantle lobe double arrowsheads<br />
setae muscles + + - mantle lobe sm<br />
setae pouch<br />
musculature<br />
+ + - mantle lobe arrowheads<br />
U-shaped muscle + + +<br />
(ventral mantle<br />
muscle)<br />
mantle lobe<br />
empty arrows<br />
Page 12 of 14<br />
(page number not for citation purposes)
50 Chapter II<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
F-actin labelling, confocal laserscanning microscopy<br />
(CLSM), and 3D reconstruction<br />
Prior to staining, larvae were washed thrice for 15 min in<br />
PB and incubated for 1 h in PB containing 0.1% Triton X-<br />
100 (Sigma-Aldrich) to permeabilize the tissue. Then, the<br />
specimens were incubated in 1:40 diluted Alexa Fluor 488<br />
phalloidin (Invitrogen, Molecular Probes, Eugene, OR,<br />
USA) and 3 μg/ml DAPI (Invitrogen) in the permeabilization<br />
solution overnight at 4°C. Subsequently, specimens<br />
were washed thrice for 15 min in 0.1 M PB and embedded<br />
in Fluoromount G (Southern Biotech, Birmingham, AL,<br />
USA) on glass slides. The same procedure was used for<br />
juveniles and adults, with the addition of a decalcifying<br />
step using 0.05 M EGTA (Sigma-Aldrich) at room temperature<br />
overnight prior to permeabilization and staining.<br />
Negative controls omitting the phalloidin dye were performed<br />
on all species in order to avoid potential misinterpretations<br />
caused by autofluorescence.<br />
The samples were analysed with a Leica DM RXE 6 TL fluorescence<br />
microscope equipped with a TCS SP2 AOBS<br />
laserscanning device (Leica Microsystems, Wetzlar, Germany).<br />
Animals were scanned at intervals of 0.49 μm or<br />
0.64 μm, respectively, and the resulting image stacks were<br />
merged into maximum projection images. Photoshop<br />
CS3 (Adobe, San Jose, CA, USA) was used to create overlay<br />
images of CLSM and light micrographs and for assembling<br />
the figure plates. 3D reconstruction was performed<br />
on CLSM datasets using volume rendering algorithms of<br />
the graphics software Imaris 5.7.2 (Bitplane, Zurich, Switzerland).<br />
Competing interests<br />
The authors declare that they have no competing interests.<br />
Authors' contributions<br />
AA performed research and drafted the manuscript. AW<br />
designed and coordinated research, performed the SEM<br />
analysis, and contributed significantly to the writing of<br />
the manuscript. Both authors read and approved the final<br />
version of the manuscript.<br />
Additional material<br />
Additional file 1<br />
Larval musculature of Argyrotheca cordata. Movie of a confocal scan<br />
through a fully developed larva of Argyrotheca cordata to illustrate the<br />
three-dimensional arrangement of the larval musculature.<br />
Click here for file<br />
[http://www.biomedcentral.com/content/supplementary/1742-<br />
9994-6-3-S1.mpg]<br />
Acknowledgements<br />
We are grateful to Henrike Semmler (Copenhagen) for rearing and fixing<br />
Terebratalia larvae during the Comparative Invertebrate Embryology class<br />
2006 at the Friday Harbor Laboratories and for comments on an early draft<br />
of the manuscript. We further thank the divers and the staff of the Marine<br />
Biological Station Banyuls-sur-mer for collecting the coralligène and for<br />
providing laboratory space. Scott Santagata (Brookville, New York) is<br />
thanked for comments on the manuscript and Jana Hoffmann (Berlin, Germany)<br />
for providing access to some of the classic literature. The valuable<br />
comments of an anonymous reviewer helped to improve the manuscript.<br />
This study was funded by the Danish Agency for Science, Technology and<br />
Innovation (grant no. 645-06-0294 to AW) and the Danish Research<br />
Agency (grant no. 21-04-0356 to AW). Research in the lab of A. Wanninger<br />
is further supported by the EU-funded Marie Curie Network MOLMORPH<br />
(contract grant number MEST-CT-2005-020542).<br />
References<br />
1. Williams A, Rowell AJ: Evolution and Phylogeny. In Treatise on<br />
Invertebrate Paleontology, Part H, Brachiopoda Volume 1. Edited by:<br />
Moore RC. Lawrence, Kansas: The Geological Society of America and<br />
The University of Kansas; 1965:164-199.<br />
2. Logan A: Geographic distribution of extant articulated brachiopods.<br />
In Treatise on Invertebrate Paleontology, Part H, Brachiopoda,<br />
Revised Volume 6. Edited by: Selden PA. Boulder, Colorado, and Lawrence,<br />
Kansas: The Geological Society of America, Inc. and The University<br />
of Kansas; 2007:3082-3115.<br />
3. Stechmann A, Schlegel M: Analysis of the complete mitochondrial<br />
DNA sequence of the brachiopod Terebratulina retusa<br />
places Brachiopoda within the protostomes. Proc R Soc Lond B<br />
Biol Sci 1999, 266:2043-2052.<br />
4. Williams A, Carlson SJ, Brunton CHC, Holmer LE, Popov L: A supraordinal<br />
classification of the Brachiopoda. Proc R Soc Lond B Biol<br />
Sci 1996, 351:1171-1193.<br />
5. Nielsen C: The phylogenetic position of Entoprocta, Ectoprocta,<br />
Phoronida, and Brachiopoda. Integr Comp Biol 2002,<br />
42:685-691.<br />
6. Lüter C, Bartolomaeus T: The phylogenetic position of Brachiopoda<br />
– a comparison of morphological and molecular data.<br />
Zool Scr 1997, 26:245-253.<br />
7. Dunn CW, Hejnol A, Matus DQ, Pang K, Browne WE, Smith SA,<br />
Seaver E, Rouse GW, Obst M, Edgecombe GD, et al.: Broad phylogenomic<br />
sampling improves resolution of the animal tree of<br />
life. Nature 2008, 452:745-749.<br />
8. Helmkampf M, Bruchhaus I, Hausdorf B: Multigene analysis of<br />
lophophorate and chaetognath phylogenetic relationships.<br />
Mol Phylogenet Evol 2008, 46:206-214.<br />
9. Mallatt J, Winchell CJ: Testing the new animal phylogeny: First<br />
use of combined large-subunit and small-subunit rRNA gene<br />
sequences to classify the protostomes. Mol Biol Evol 2002,<br />
19:289-301.<br />
10. Passamaneck Y, Halanych KM: Lophotrochozoan phylogeny<br />
assessed with LSU and SSU data: Evidence of lophophorate<br />
polyphyly. Mol Phylogenet Evol 2006, 40:20-28.<br />
11. Peterson KJ, Eernisse DJ: Animal phylogeny and the ancestry of<br />
bilaterians: inferences from morphology and 18S rDNA gene<br />
sequences. Evol Dev 2001, 3:170-205.<br />
12. Cohen BL: Monophyly of brachiopods and phoronids: reconciliation<br />
of molecular evidence with Linnaean classification<br />
(the subphylum Phoroniformea nov.). Proc R Soc Lond B Biol Sci<br />
2000, 267:225-231.<br />
13. Cohen BL, Weydmann A: Molecular evidence that phoronids<br />
are a subtaxon of brachiopods (Brachiopoda: Phoronata)<br />
and that genetic divergence of metazoan phyla began long<br />
before the early Cambrian. Org Divers Evol 2005, 5:253-273.<br />
14. Kowalevski AO: Observations sur le développement des brachiopodes<br />
(Analyse par Oehlert et Deniker). Arch Zool Exp Gen<br />
1883, Series 2:57-76.<br />
Page 13 of 14<br />
(page number not for citation purposes)
Chapter II<br />
51<br />
Frontiers in Zoology 2009, 6:3<br />
http://www.frontiersinzoology.com/content/6/1/3<br />
15. Plenk H: Die Entwicklung von Cistella (Argiope) neapolitana.<br />
Ein Beitrag zur Entwicklungsgeschichte der Brachiopoden<br />
(1. Mitteilung). Arb zool Inst Univ Wien 1913, 20:93-108.<br />
16. Chuang SH: The embryonic, larval and early postlarval development<br />
of the terebratellid brachiopod Calloria inconspicua<br />
(Sowerby). J R Soc NZ 1996, 26:119-137.<br />
17. Long JA: The embryology of three species representing three<br />
superfamilies of articulate Brachiopoda. University of Washington,<br />
<strong>PhD</strong> Thesis; 1964.<br />
18. Lüter C: Embryonic and larval development of Calloria inconspicua<br />
(Brachiopoda, Terebratellidae). J R Soc NZ 1998,<br />
28:165-167.<br />
19. Grobe P, Lüter C: Reproductive cycles and larval morphology<br />
of three Recent species of Argyrotheca (Terebratellacea: Brachiopoda)<br />
from Mediterranean submarine caves. Mar Biol<br />
1999, 134:595-600.<br />
20. Lüter C: Anatomy. In Treatise on Invertebrate Paleontology, Part H,<br />
Brachiopoda, Revised Volume 6. Edited by: Selden PA. Boulder, Colorado,<br />
and Lawrence, Kansas: The Geological Society of America, Inc.<br />
and The University of Kansas; 2007:2321-2355.<br />
21. Williams A, Rowell AJ: Brachiopod Anatomy. In Treatise on Invertebrate<br />
Paleontology, Part H, Brachiopoda Volume 1. Edited by: Moore<br />
RC. Lawrence, Kansas: Geological Society of America and University<br />
of Kansas Press; 1965:6-57.<br />
22. Hooge MD, Tyler S: Concordance of molecular and morphological<br />
data: The example of the Acoela. Integr Comp Biol 2006,<br />
46:118-124.<br />
23. Wanninger A: Myo-anatomy of juvenile and adult loxosomatid<br />
entoprocta and the use of muscular body plans for phylogenetic<br />
inferences. J Morphol 2004, 261:249-257.<br />
24. Santagata S: Structure and metamorphic remodeling of the<br />
larval nervous system and musculature of Phoronis pallida<br />
(Phoronida). Evol Dev 2002, 4:28-42.<br />
25. Gruhl A: Muscular systems in gymnolaemate bryozoan larvae<br />
(Bryozoa: Gymnolaemata). Zoomorphology 2008, 127:143-159.<br />
26. Santagata S: Larval development of Phoronis pallida (Phoronida):<br />
Implications for morphological convergence and divergence<br />
among larval body plans. J Morphol 2004, 259:347-358.<br />
27. Santagata S, Zimmer RL: Comparison of the neuromuscular systems<br />
among actinotroch larvae: systematic and evolutionary<br />
implications. Evol Dev 2002, 4:43-54.<br />
28. Santagata S: The morphology and evolutionary significance of<br />
the ciliary fields and musculature among marine bryozoan<br />
larvae. J Morphol 2008, 269:349-364.<br />
29. Lüter C: Ultrastructure of larval and adult setae of Brachiopoda.<br />
Zool Anz 2000, 239:75-90.<br />
30. Shipley AE: On the structure and development of Argiope. Mitt<br />
zool Stat Neapel 1883, 4:494-520.<br />
31. Chuang SH: Larval development in Discinisca (inarticulate brachiopod).<br />
Am Zool 1977, 17:39-53.<br />
32. Stricker SA, Reed CG: The ontogeny of shell secretion in Terebratalia<br />
transversa (Brachiopoda, Articulata). 1. Development<br />
of the mantle. J Morphol 1985, 183:233-250.<br />
33. Lüter C: Zur Ultrastruktur, Ontogenese und Phylogenie der Brachiopoda<br />
Göttingen: Cuvillier Verlag; 1998.<br />
34. James MA, Ansell AD, Collins MJ, Curry GB, Peck LS, Rhodes MC:<br />
Biology of living brachiopods. Adv Mar Biol 1992, 28:175-387.<br />
35. Stricker SA, Reed CG: The ontogeny of shell secretion in Terebratalia<br />
transversa (Brachiopoda, Articulata). 2. Formation of<br />
the protegulum and juvenile shell. J Morphol 1985, 183:251-271.<br />
36. Reed CG, Cloney RA: Brachiopod tentacles – ultrastructure<br />
and functional significance of connective-tissue and myoepithelial<br />
cells in Terebratalia. Cell Tissue Res 1977, 185:17-42.<br />
37. Eshleman WP, Wilkens JL: Actomyosin ATPase activities in the<br />
brachiopod Terebratalia transversa. Can J Zool 1979,<br />
57:1944-1949.<br />
38. Strathmann RR: Evolution and loss of feeding larval stages of<br />
marine invertebrates. Evolution 1978, 32:894-906.<br />
39. Pennington JT, Stricker SA: Phylum Brachiopoda. In Atlas of<br />
Marine Invertebrate Larvae Edited by: Young CM. San Diego: Academic<br />
Press; 2002:441-461.<br />
40. de Lacaze-Duthiers FJH: Histoire naturelle des brachiopodes<br />
vivants de la Mediterranée. I. Histoire naturelle de la Thecidie<br />
(Thecidium mediterraneum). Ann Sci Nat Zool (serie 4) 1861,<br />
15:259-330.<br />
41. Hill SD, Boyer BC: Phalloidin labeling of developing muscle in<br />
embryos of the polychaete Capitella sp. I. Biol Bull 2001,<br />
201:257-258.<br />
42. Reiter D, Boyer B, Ladurner P, Mair G, Salvenmoser W, Rieger R:<br />
Differentiation of the body wall musculature in Macrostomum<br />
hystricinum marinum and Hoploplana inquilina<br />
(Plathelminthes) as models for muscle development in<br />
lower Spiralia. Dev Genes Evol 1996, 205:410-423.<br />
43. Fuchs J, Bright M, Funch P, Wanninger A: Immunocytochemistry<br />
of the neuromuscular systems of Loxosomella vivipara and<br />
Loxosomella parguerensis (Entoprocta: Loxosomatidae). J<br />
Morphol 2006, 267:866-883.<br />
44. McDougall C, Chen W-C, Shimeld S, Ferrier D: The development<br />
of the larval nervous system, musculature and ciliary bands<br />
of Pomatoceros lamarckii (Annelida): heterochrony in polychaetes.<br />
Front Zool 2006, 3:16.<br />
45. Nielsen C, Haszprunar G, Ruthensteiner B, Wanninger A: Early<br />
development of the aplacophoran mollusc Chaetoderma.<br />
Acta Zool 2007, 88:231-247.<br />
46. Wanninger A, Haszprunar G: Chiton myogenesis: Perspectives<br />
for the development and evolution of larval and adult muscle<br />
systems in molluscs. J Morphol 2002, 251:103-113.<br />
47. Wanninger A, Ruthensteiner B, Lobenwein S, Salvenmoser W, Dictus<br />
WJAG, Haszprunar G: Development of the musculature in the<br />
limpet Patella (Mollusca, Patellogastropoda). Dev Genes Evol<br />
1999, 209:226-238.<br />
Publish with BioMed Central and every<br />
scientist can read your work free of charge<br />
"BioMed Central will be the most significant development for<br />
disseminating the results of biomedical research in our lifetime."<br />
Sir Paul Nurse, Cancer Research UK<br />
Your research papers will be:<br />
available free of charge to the entire biomedical community<br />
peer reviewed and published immediately upon acceptance<br />
cited in PubMed and archived on PubMed Central<br />
yours — you keep the copyright<br />
Submit your manuscript here:<br />
http://www.biomedcentral.com/info/publishing_adv.asp<br />
BioMedcentral<br />
Page 14 of 14<br />
(page number not for citation purposes)
52 Chapter III<br />
Chapter III<br />
Altenburger, A. & Wanninger, A. 2010 Neuromuscular development<br />
in Novocrania anomala: evidence for the presence of serotonin<br />
and a spiralian-like apical organ in lecithotrophic brachiopod<br />
larvae. Evolution & Development 12: 16-24
Chapter III<br />
53<br />
EVOLUTION & DEVELOPMENT 12:1, 16–24 (2010)<br />
DOI: 10.1111/j.1525-142X.2009.00387.x<br />
Neuromuscular development in Novocrania anomala: evidence for the<br />
presence of serotonin and a spiralian-like apical organ in lecithotrophic<br />
brachiopod larvae<br />
Andreas Altenburger and Andreas Wanninger <br />
Department of Biology, Research Group for Comparative Zoology, University of Copenhagen, Universitetsparken 15,<br />
DK-2100 Copenhagen Ø, Denmark<br />
Author for correspondence (email: awanninger@bio.ku.dk)<br />
SUMMARY The phylogenetic position of Brachiopoda remains<br />
unsettled, and only few recent data on brachiopod<br />
organogenesis are currently available. In order to contribute<br />
data to questions concerning brachiopod ontogeny and evolution<br />
we investigated nervous and muscle system development<br />
in the craniiform (inarticulate) brachiopod Novocrania<br />
anomala. Larvae of this species are lecithotrophic and have<br />
a bilobed body with three pairs of dorsal setal bundles that<br />
emerge from the posterior lobe. Fully developed larvae exhibit<br />
a network of setae pouch muscles as well as medioventral<br />
longitudinal and transversal muscles. After settlement, the<br />
anterior and posterior adductor muscles and delicate mantle<br />
retractor muscles begin to form. Comparison of the larval<br />
muscular system of Novocrania anomala with that of<br />
rhynchonelliform (articulate) brachiopod larvae shows that<br />
the former has a much simpler muscular organization. The<br />
first signal of serotonin-like immunoreactivity appears in fully<br />
developed Novocrania anomala larvae, which have an apical<br />
organ that consists of four flask-shaped cells and two ventral<br />
neurites. These ventral neurites do not stain positively for the<br />
axonal marker a-tubulin in the larval stages. In the juveniles,<br />
the nervous system stained by a-tubulin is characterized by<br />
two ventral neurite bundles with three commissures. Our data<br />
are the first direct proof for the presence of an immunoreactive<br />
neurotransmitter in lecithotrophic brachiopod larvae and<br />
demonstrate the existence of flask-shaped serotonergic cells<br />
in the brachiopod larval apical organ, thus significantly<br />
increasing the probability that this cell type was part of the<br />
bauplan of the larvae of the last common lophotrochozoan<br />
ancestor.<br />
INTRODUCTION<br />
The phylogenetic position of Brachiopoda remains unresolved,<br />
although most molecular analyses agree on their inclusion<br />
within Lophotrochozoa (Hejnol et al. 2009; Paps et al.<br />
2009). Alternatively, some recent works support the more<br />
traditional view that Brachiopoda clusters with Ectoprocta<br />
and Phoronida to form the Lophophorata, the direct sistergroup<br />
of Spiralia (Trochozoa) (Gee 1995; Nielsen 2002; Halanych<br />
2004). Current brachiopod internal phylogeny suggests<br />
division of the phylum into the three clades Linguliformea,<br />
Craniiformea, and Rhynchonelliformea (Williams et al. 1996).<br />
Craniiform brachiopods share morphological traits with both<br />
linguliforms and rhynchonelliforms. For example, craniiforms<br />
and linguliforms possess a circumferential mantle cavity, a<br />
muscle system with oblique muscles, and two pairs of shell<br />
adductors, a transitional median tentacle during lophophore<br />
development and a median division of the brachial canals into<br />
two separate cavities within the lophophore. Craniiforms and<br />
rhynchonelliformes exhibit a proteinaceous calcitic shell, a<br />
16<br />
single row of tentacles on a trocholophous lophophore,<br />
gonads suspended in the mantle sinus, and lecithotrophic<br />
larvae (Rowell 1960; Atkins and Rudwick 1962; Williams<br />
et al. 1996).<br />
Experimental embryology has shown that the animal half<br />
of the egg forms the ectodermal epithelium of the apical lobe,<br />
whereas the vegetal half forms endoderm, mesoderm, and the<br />
ectoderm of the mantle lobe in Novocrania anomala (Mu¨ ller<br />
1776) (previously assigned to various genera and thus also<br />
referred to in the literature as Crania anomala or Neocrania<br />
anomala, respectively) (Lee and Brunton 1986, 2001; Freeman<br />
and Lundelius 1999; Freeman 2000; Holmer 2001; Cohen et<br />
al. 2008). During metamorphosis, both the ventral and the<br />
dorsal valve are formed from the dorsal epithelium of the<br />
larva (Nielsen 1991).<br />
Recent immunocytochemical studies have revealed the almost<br />
universal occurrence of an apical organ that contains<br />
flask-shaped cells in larvae of Annelida, Mollusca, Sipuncula,<br />
Entoprocta, and Platyhelminthes (see Wanninger 2009 for<br />
review). These flask-shaped cells express serotonin-like<br />
& 2010 Wiley Periodicals, Inc.
54 Chapter III<br />
Altenburger and Wanninger<br />
immunoreactivity and may also show FMRFamidergic<br />
immunoreactivity. The wide occurrence of serotonin indicates<br />
that this neurotransmitter was part of the ancestral metazoan<br />
nervous system (Hay-Schmidt 2000). Surprisingly, neither<br />
serotonin-like immunoreactivity nor the existence of flaskshaped<br />
cells have hitherto been proven for lecithotrophic larvae<br />
of any brachiopod clade, thus leaving a significant gap in<br />
our understanding of the evolution of the brachiopod nervous<br />
system and the origin of this cell type within the lophophorates.<br />
Accordingly, we provide herein the first thorough<br />
immunocytochemical study on neurogenesis in a brachiopod<br />
with a lecithotrophic larva, the craniiform Novocrania anomala,<br />
and compare our findings with data on other lophotrochozoan<br />
phyla. In our general quest to shed light on<br />
brachiopod organogenesis, we also present data on Novocrania<br />
anomala myogenesis, which for the first time allows conclusive<br />
comparisons between the muscular systems of<br />
craniiform and rhynchonelliform brachiopod larvae and thus<br />
contributes to answering questions concerning the ancestral<br />
muscular bodyplan of brachiopod larvae.<br />
MATERIALS AND METHODS<br />
Animal collection, breeding, and fixation<br />
Rocks with attached adults of Novocrania anomala where obtained<br />
by dredging in the vicinity of the Sven Love´ n Centre for Marine<br />
Sciences, Gullmarsfjord, Sweden (58115 0 921 00 N, 11125 0 103 00 E) in<br />
October 2007 and September 2008. The rocks were maintained in<br />
the laboratory in running seawater and adults were removed and<br />
dissected for gametes. For artificial fertilization, eggs and sperm<br />
were removed from the gonads with pulled glass pipettes and<br />
placed in separate glass beakers with filtered seawater at ambient<br />
seawater temperature (141C). The water containing the eggs was<br />
changed at least four times to wash off follicle cells and superfluous<br />
gonad tissue. Eggs were regularly checked for germinal vesicle<br />
breakdown and sperm cells were checked for motility under a<br />
compound microscope. After approximately 12 h, 2 ml of a highly<br />
diluted sperm suspension (testes of three to five adults in approximately<br />
100 ml filtered sea water) were added to the beakers containing<br />
eggs. Developing larvae were fixed at various stages after<br />
fertilization (from 34 h post-fertilization [hpf] to 17 days post-settlement)<br />
in 4% paraformaldehyde in 0.1 M phosphate buffer (PB)<br />
for 90 min. Thereafter, larvae were washed three times for 15 min<br />
each in 0.1 M PB and finally stored in 0.1 M PB containing 0.1%<br />
NaN 3 at 41C.<br />
Immunocytochemistry, confocal laserscanning<br />
microscopy (CLSM), and three-dimensional (3D)<br />
reconstruction<br />
Before staining, larvae were washed thrice for 15 min each in PB<br />
and incubated for 1 h in PB containing 0.2% Triton X-100<br />
(Sigma-Aldrich, St. Louis, MO, USA) at room temperature to<br />
permeabilize the tissue. For F-actin staining, specimens were left<br />
overnight at 41C in0.1M PB containing 0.2% Triton X-100 and<br />
1:40 diluted Alexa Fluor 488 phalloidin (Invitrogen, Molecular<br />
Probes, Eugene, OR, USA). For serotonin and a-tubulin staining,<br />
specimens were first incubated overnight at 41C in 6% normal goat<br />
serum in 0.1 M PB and 0.2% Triton X-100 (blocking solution).<br />
Second, specimens were incubated for 24 h at 41C in blocking solution<br />
containing either a 1:800 diluted polyclonal primary serotonin<br />
antibody (Zymed, Carlton Court, CA, USA), or a 1:500<br />
diluted monoclonal primary acetylated a-tubulin antibody (Sigma-<br />
Aldrich). Third, specimens were washed in the permeabilization<br />
solution overnight at 41C with four changes. Then, the secondary<br />
antibodies (either Alexa Fluor 633-conjugated goat anti-rabbit,<br />
Invitrogen or TRITC-conjugated goat anti-rabbit, Sigma-Aldrich)<br />
were added in a 1:300 dilution to the blocking solution and the<br />
samples were incubated for 24 h. Subsequently, the specimens were<br />
washed three times for 15 min each in 0.1 M PB and embedded in<br />
Fluoromount G (Southern Biotech, Birmingham, AL, USA) on<br />
glass slides. Negative controls omitting either the phalloidin dye or<br />
the respective secondary antibody were performed in order to test<br />
for signal specificity and rendered no signal. The samples were<br />
analyzed with a Leica DM RXE 6 TL fluorescence microscope<br />
equipped with a TCS SP2 AOBS laserscanning device (Leica Microsystems,<br />
Wetzlar, Germany). Animals were scanned with 0.16–<br />
0.49 mm step size, and the resulting image stacks were merged into<br />
maximum projection images. In addition, light micrographs were<br />
recorded to allow overlay with the CLSM images for exact orientation<br />
and localization of the muscle and nervous systems within<br />
the animals. Adobe Photoshop CS3 software (Adobe, San Jose,<br />
CA, USA) was used to create overlay images and for assembling<br />
the figure plates. The sketch drawings were generated with Adobe<br />
Illustrator CS3 (Adobe), and the 3D reconstructions were created<br />
with the Imaris imaging software version 5.7.2 (Bitplane, Zu¨ rich,<br />
Switzerland) based on the CLSM image stacks.<br />
RESULTS<br />
Brachiopod neuromuscular development 17<br />
Myogenesis<br />
The first signals of F-actin were found in the setae pouches of<br />
bilobed larvae at the onset of setae formation. The six setae<br />
pouches are distributed in pairs along the dorsal ridge of the<br />
posterior lobe (Fig. 1A). As the setae grow, the setae pouch<br />
muscles develop further into spherical systems (Figs. 1, B and<br />
G–I and 2A). Later in development, the setae pouch muscles<br />
get interconnected by two bundles of medioventral longitudinal<br />
muscles, which run ventrally from anterior to posterior<br />
(Figs. 1, C and D and G–I and 2A). The medioventral longitudinal<br />
muscle strands get interconnected by transversal<br />
muscles (Fig. 1, B–D and G–I) that are distributed homogenously<br />
in early stages (Fig. 1B) and concentrate into three<br />
bundles in later stages (Fig. 1D). Accordingly, the metamorphic<br />
competent larva has setae pouch muscles, medioventral<br />
longitudinal muscles, and transversal muscles. During metamorphosis,<br />
the larval musculature is replaced by the juvenile<br />
musculature, which most likely develops entirely de novo, that<br />
is, independent of the larval muscle systems (Fig. 1E). The
Chapter III<br />
55<br />
18 EVOLUTION & DEVELOPMENT Vol. 12, No. 1, January--February 2010<br />
Fig. 1. Muscle development in Novocrania anomala. Overlay of maximum projection micrographs from phalloidin staining and light<br />
micrographs. Anterior faces upwards and scale bars equal 50 mm. (A) Larva with anterior lobe (AL), posterior lobe (PL), and early signs of<br />
F-actin in the three pairs of setae pouches (arrows) along the dorsal ridge of the posterior lobe. (B) Larva with setae (se), anterior lobe (AL),<br />
posterior lobe (PL), setae pouch muscles (arrows), homogenously distributed transversal muscles (asterisks), and a distinct F-actin-rich area<br />
(arrowheads), which might be involved in cementing the larva to the substrate during settlement. (C) Later larval stage with setae pouch<br />
muscles (arrows), medioventral longitudinal muscles (empty arrows), and F-actin-rich area (arrowhead) on the dorsal side. (D) Metamorphic<br />
competent larva in ventral view with setae (se) and setae pouch muscles (arrows), which are ventrally interconnected by two strands<br />
of medioventral longitudinal muscles (empty arrows). The medioventral longitudinal muscles are interconnected by transversal muscles,<br />
which at this stage are concentrated into three bundles (asterisks). (E) Specimen during metamorphosis with remnants of larval setae pouch<br />
muscles (arrows) and larval medioventral longitudinal muscles (empty arrows), which are most probably undergoing resorption. The adult<br />
anterior adductor muscles (aad) start to develop. (F) Juvenile with mantle margin muscles (mm), anterior adductor muscle (aad), oblique<br />
muscle (ob), and posterior adductor muscles (pad). (G–I) Three-dimensional reconstruction of the dataset shown in (D). (G) Ventral view of<br />
the musculature of a fully developed larva with medioventral longitudinal muscles (red), setae pouch muscles (yellow), and transversal<br />
muscle (asterisk). (H) Same specimen as in (G), anterior view. (I) Same specimen as in (G), dorsal view.
56 Chapter III<br />
Altenburger and Wanninger<br />
Brachiopod neuromuscular development 19<br />
Fig. 2. Semischematic representation of<br />
the larval musculature of craniiform and<br />
rhynchonelliform brachiopods. (A) Musculature<br />
of Novocrania anomala with<br />
setae pouch muscles (red circles), medioventral<br />
longitudinal muscles (white), and<br />
transversal muscles (yellow-grey). Size of<br />
the specimen is approximately 150 mm.<br />
(B) Musculature of Argyrotheca cordata<br />
based on Altenburger and Wanninger<br />
(2009) with pedicle muscles (beige), longitudinal<br />
muscles (orange), central mantle<br />
muscles (brown), U-shaped muscle<br />
(green), setae pouch muscles (red circles),<br />
circular mantle muscle (light blue), serial<br />
mantle muscles (dark orange), setae muscles<br />
(purple), apical longitudinal muscles<br />
(dark blue), and apical transversal muscle<br />
(yellow). Size of the specimen is approximately<br />
280 mm.<br />
juvenile musculature comprises mantle margin muscles,<br />
oblique muscles, as well as anterior and posterior adductor<br />
muscles (Fig. 1F).<br />
Neurogenesis<br />
The first signals of serotonin-like immunoreactivity appear in<br />
fully developed, metamorphic competent, bilobed larvae at<br />
approximately 86 hpf (Table 1). At this stage, four flaskshaped<br />
cells are present in the anterior-most part of the apical<br />
lobe (Fig. 3, A–D). They are oriented in different directions<br />
with only one pointing toward the apical pole of the larva.<br />
The flask-shaped cells are connected to two ventral neurites<br />
that extend posteriorly (Fig. 3, A–D). The flask-shaped cells<br />
are lost during metamorphosis, and early juveniles have two<br />
ventral neurites that project from the anterior lobe into the<br />
posterior lobe (Fig. 3E). During subsequent development, the<br />
ventral neurites become interconnected by a median commissure<br />
in the mid-part of the juvenile (Fig. 3F).<br />
The axonal marker a-tubulin is first expressed in juveniles 5<br />
days after metamorphosis (Fig. 4A). Two solid neurite bundles<br />
develop ventrolaterally in the anterior lobe of the juvenile and<br />
subsequently grow in posterior direction into the posterior lobe<br />
(Fig. 4B). Later in development, these neurite bundles close by<br />
an anterior and a posterior commissure, and the median commissure<br />
is established (Fig. 4, C–F). Serially arranged mantle<br />
neurites extend from the anterior part of the ventral neurite<br />
bundles in a lateral direction toward the mantle margin of<br />
the juvenile (Fig. 4, B–F). Comparison of the position of the<br />
a-tubulin signal in the juvenile and the serotonin-like signal in<br />
the larva suggests that the larval ventral neurites are the earliest<br />
neurites of the future ventral neurite bundles of the juvenile.<br />
Table 1. Landmarks of Novocrania anomala development at 141C<br />
Age (hours post<br />
fertilization)<br />
Gross morphology<br />
Myoanatomy as inferred by<br />
F-actin staining<br />
3–4 First cleavage No signal No signal<br />
26–30 Swimming, spherical gastrula No signal No signal<br />
42–49 Swimming, elongated gastrula No signal No signal<br />
65–73 Swimming, bilobed larva with<br />
setae starting to develop<br />
First signals of actin in setae<br />
pouches (Fig. 1A)<br />
No signal<br />
86–96 Fully established, swimming,<br />
bilobed larva with long setae<br />
168 Settled juvenile after metamorphosis<br />
Fully developed larval musculature<br />
with setae pouch muscles, longitudinal<br />
muscles, and transversal muscles<br />
(Fig. 1D)<br />
Juvenile with mantle margin muscles,<br />
anterior adductors, and posterior<br />
adductors (Fig. 1F)<br />
Neuroanatomy as inferred by<br />
antibody staining<br />
Larval nervous system with four<br />
flask-shaped cells in the apical organ<br />
and two ventral neurites (Fig.<br />
3, B–D)<br />
Juvenile with two ventral neurite<br />
bundles, commissures, and serially<br />
arranged neurites (Figs. 3, E and<br />
F and 4, A–F)
Chapter III<br />
57<br />
20 EVOLUTION & DEVELOPMENT Vol. 12, No. 1, January--February 2010<br />
Fig. 3. Development of the serotonergic<br />
nervous system in Novocrania anomala.<br />
(A, B, E, and F) Overlay of maximum<br />
projection micrographs of serotonin<br />
staining and light micrographs. (C and<br />
D) Three-dimensional reconstruction of<br />
the dataset shown in B. Anterior faces<br />
upwards and scale bars equal 50 mm. (A<br />
and B) Metamorphic competent larva<br />
with three pairs of setae bundles (se) and<br />
four flask-shaped serotonergic cells (asterisks)<br />
in the anterior part of the apical<br />
lobe (AL), as well as two ventral<br />
serotonergic neurites (arrows) running<br />
from the apical lobe toward the posterior<br />
lobe (PL). The stage in (A) is slightly<br />
younger than that depicted in (B). (C and<br />
D) Same dataset as in (B) with four flaskshaped<br />
serotonergic cells (red) and two<br />
ventral neurites, which are interconnected<br />
anteriorly (yellow). (C) Ventral view. (D)<br />
Lateral view. The flask-shape is visible<br />
only in one cell due to the different position<br />
of the cells. (E) Juvenile during<br />
metamorphosis with two ventral neurites<br />
(arrows), which run from the region of<br />
the former anterior lobe (AL) into the<br />
region of the former posterior lobe (PL).<br />
Larval setae (se) and juvenile shell (s) are<br />
present. (F) Later stage of a juvenile with<br />
two ventral neurites (arrows) which are<br />
interconnected by a median commissure<br />
(mco).<br />
DISCUSSION<br />
Comparative brachiopod myoanatomy<br />
The musculature of fully developed Novocrania anomala larvae<br />
consists of setae pouch muscles, the medioventral longitudinal<br />
muscles that interconnect these setae pouch muscles,<br />
and transversal muscles that interconnect the medioventral<br />
longitudinal muscles (Table 1 and Fig. 2A). This relatively<br />
simple muscular organization differs significantly from that of<br />
articulate brachiopod larvae, which comprises pedicle muscles,<br />
longitudinal muscles, a circular mantle muscle, central<br />
mantle muscles, a U-shaped muscle, serially arranged mantle<br />
muscles, setae muscles, setae pouch muscles, apical longitudinal<br />
muscles, and an apical transversal muscle (Fig. 2B; see<br />
also Altenburger and Wanninger 2009). Unfortunately, very<br />
little is known about brachiopod larval ecology and behavior
58 Chapter III<br />
Altenburger and Wanninger<br />
Brachiopod neuromuscular development 21<br />
Fig. 4. Development of the nervous system<br />
in Novocrania anomala as revealed by<br />
acetylated a-tubulin staining. (A–D)<br />
Overlay of maximum projection micrograph<br />
of a-tubulin staining and light micrograph.<br />
(E and F) Three-dimensional<br />
reconstructions of the dataset shown in<br />
(D). Anterior faces upwards and scale<br />
bars equal 50 mm. (A) First a-tubulin signal<br />
in a juvenile 5 days after metamorphosis.<br />
The former larval apical lobe<br />
(AL) and posterior lobe (PL) are still<br />
visible under the shell (s) of the juvenile.<br />
Two ventral neurite bundles develop in<br />
the anterior lobe (arrows). The juvenile<br />
body is still covered by larval cilia (ci).<br />
Some serially arranged neurites (sn) extend<br />
inwards from the ventral neurite<br />
bundles. (B) The ventral neurite bundles<br />
(arrows) elongate further in posterior direction.<br />
A median commissure (mco)<br />
starts to form. From the anterior portion<br />
of the ventral neurite bundles, serially arranged<br />
mantle neurites (smn) extend distally<br />
outwards, and serially arranged<br />
neurites (sn) extend inwards. The cilia of<br />
the juvenile gut (gu) are visible in the<br />
median region of the juvenile. (C) Juvenile<br />
with the same structures as in (B).<br />
The median commissure (mco) is closed<br />
and the ventral neurite bundles (arrows)<br />
have fused anteriorly to form the anterior<br />
commissure (aco). (D) Neural anatomy<br />
of a juvenile 17 days after metamorphosis<br />
with an anterior commissure (aco), a median<br />
commissure (mco), and a posterior<br />
commissure (pco) that interconnect the<br />
ventral neural bundles (arrows). In addition,<br />
the serially arranged mantle neurites<br />
(smn), which extend toward the edge of<br />
the juvenile mantle, are visible. (E) Threedimensional<br />
reconstruction of the dataset<br />
shown in (D), dorsal view. (F) Three-dimensional<br />
reconstruction of the dataset<br />
shown in (D). Postero-dorsal view demonstrating<br />
that the ventral neurite bundles<br />
(yellow) and the serially arranged<br />
mantle neurites (green) bend ventrally.<br />
(James et al. 1992). Rhynchonelliform larvae show a change<br />
from positive to negative phototactism when reaching metamorphic<br />
competence. In laboratory cultures, they swim in the<br />
culture dish with the anterior lobe or the ventral side of the<br />
body repeatedly forming contact with the bottom of the dish,<br />
probably probing for a suitable place for settlement (Chuang<br />
1996). We observed a similar behavior in Novocrania anomala<br />
larvae before metamorphosis.<br />
At the current state of knowledge, it remains difficult to<br />
relate the differences in larval myoanatomy to aspects concerning<br />
the ecology of the respective brachiopod larvae, because<br />
the latter remains virtually unknown (James et al. 1992).<br />
An earlier study showed that larvae of Novocrania anomala<br />
are able to settle 4 days after fertilization (Nielsen 1991), although<br />
we observed this behavior only in 7-day-old larvae.<br />
Rhynchonelliform brachiopods are known to settle after 3
Chapter III<br />
59<br />
22 EVOLUTION & DEVELOPMENT Vol. 12, No. 1, January--February 2010<br />
(Terebratulina retusa), 5–14 (Terebratalia transversa) (own<br />
observations), or up to 160 days (Liothyrella uva) (see Peck<br />
and Robinson 1994 for listed overview). However, whether or<br />
not these differences in the planktonic lifespan of brachiopod<br />
larvae accounts for the differences in their myoanatomy remains<br />
speculative. Instead, we consider the dissimilarities in<br />
how metamorphosis is achieved in craniiform (inarticulate)<br />
and rhynchonelliform (articulate) larvae as a possible reason<br />
for this morphological variation. During metamorphosis, larvae<br />
of Novocrania anomala curl ventrally by contraction of<br />
the paired medioventral muscles and attach to the substrate<br />
via the epithelium at the posterior end of the larva. The brachial<br />
valve is then secreted by the median part of the dorsal<br />
epithelium and the pedicle valve is secreted by the attachment<br />
epithelium (Nielsen 1991). Larvae of the rhynchonelliform<br />
brachiopod T. transversa attach via a secretory product produced<br />
by the distal tip of the pedicle lobe at the posterior end<br />
of the larva. After attachment, the mantle lobe flips over the<br />
apical lobe and secretes a protegulum containing calcium<br />
carbonate (Stricker and Reed 1985; Freeman 1993).<br />
The phylogenetic relationship of craniiforms to the other<br />
brachiopod subtaxa is still controversial. Based on their lack of<br />
a valve-to-valve articulation they have traditionally been<br />
grouped together with other inarticulated groups (Williams<br />
and Rowell 1965a, b). This view is supported by molecular<br />
analyses based on 18S rDNA sequences, which either place the<br />
craniiforms within the linguliforms (Cohen 2000) or as the<br />
direct sister-group to the linguliforms (Cohen and Weydmann<br />
2005). Other morphological characters such as the presence of<br />
an anus and a lophophore without internal mineralized support<br />
underpins a close relationship of craniiform and linguliform<br />
brachiopods (Carlson 1995). However, based on the<br />
lecithotrophy of the larvae and the presence of a calcareous<br />
shell in the adults, craniiform brachiopods have been proposed<br />
to be closer related to the rhynchonelliforms rather than to<br />
the linguliforms, which have a free-swimming planktotrophic<br />
life cycle stage that closely resembles the morphology of juvenile<br />
brachiopods (Nielsen 1991). An alternative scenario proposes<br />
that lecithotrophic larvae equipped with larval setae are<br />
basal for Brachiopoda and that the swimming ‘‘paralarvae’’ of<br />
lingulids constitute a planktonic juvenile stage, thereby implying<br />
that the linguliforms have secondarily lost the lecithotrophic<br />
larva (Lu¨ ter 2001). Our data corroborates this view.<br />
The musculature of postmetamorphosic Novocrania anomala<br />
comprises anterior adductors, posterior adductors, and<br />
oblique lateral muscles. This corresponds to the musculature<br />
found in adults, which in addition have brachial protractor<br />
muscles at the base of the lophophore, an unpaired median<br />
muscle, and oblique internal muscles (Bulman 1939; Helmcke<br />
1939; Williams and Rowell 1965a, b). In the present study we<br />
found mantle retractor muscles, which had previously been<br />
undescribed for Novocrania anomala and which correspond to<br />
the respective muscles found in the rhynchonelliform brachiopods<br />
Argyrotheca cordata, Argyrotheca cistellula, and<br />
Terebratalia transversa (Altenburger and Wanninger 2009).<br />
Given the distinct differences in the larval musculature of<br />
craniiforms and rhynchonelliforms, it is difficult to infer a<br />
muscular ground pattern for brachiopod larvae. However, it<br />
appears likely that a hypothetical ancestral brachiopod larva<br />
had at least setae pouch muscles and a musculature that interconnect<br />
these setae pouch muscles.<br />
Neurogenesis<br />
The serotonergic nervous system of Novocrania anomala starts<br />
to develop in fully established larvae (see Table 1), and shows<br />
an apical organ consisting of four flask-shaped cells and two<br />
lateroventral neurites, which grow from the anterior lobe into<br />
the posterior lobe. These results constitute the first unambiguous<br />
account of the presence of an apical organ with<br />
serotonergic flask-shaped cells in a lecithotrophic brachiopod<br />
larva. Similar apical organs containing flask-shaped cells have<br />
been found in a wide range of lophotrochozoans including<br />
entoprocts (Wanninger et al. 2007), mollusks (Voronezhskaya<br />
et al. 2002; Wanninger and Haszprunar 2003), annelids<br />
(Voronezhskaya et al. 2003), and ectoprocts (Pires and Woollacott<br />
1997; Shimizu et al. 2000). The finding of an apical<br />
organ with serotonergic flask-shaped cells in a lecithotrophic<br />
brachiopod larva suggests that such an apical organ was also<br />
present in the larva of the last common lophotrochozoan ancestor<br />
(Wanninger 2009). Interestingly, such flask cells are<br />
also present in larvae of the demosponge Amphimedon queenslandica,<br />
but whether or not they express serotonin-like<br />
immunoreactivity in this species remains unknown (Sakarya<br />
et al. 2007). Accordingly, it appears that the evolution of<br />
flask-shaped cells in metazoan larvae predated the poriferan–<br />
eumetazoan split, whereby it remains possible that these cells<br />
only acquired serotonin-like immunoreactivity in the lophotrochozoan<br />
lineage. In case of such a scenario, serotoninexpressing<br />
flask cells would be a distinct apomorphy for the<br />
entire Lophotrochozoa.<br />
Similar to the vast majority of lophotrochozoan larvae,<br />
but significantly different to the situation found in the entoproct<br />
creeping-type larva and the larva of polyplacophoran<br />
mollusks, the apical organ of Novocrania anomala is comparatively<br />
simple, thus supporting the notion that a simple apical<br />
organ was present in the ‘‘ur-lophotrochozoan’’ larva,<br />
whereas a complex apical organ is likely to be a synapomorphy<br />
of a monophyletic Entoprocta1Mollusca (Tetraneuralia<br />
concept; see Wanninger 2009).<br />
A serotonergic nervous system has been described previously<br />
for planktotrophic linguliform brachiopod ‘‘paralarvae.’’<br />
There, the apical organ is located at the base of the<br />
median tentacle and comprises numerous serotonergic cells<br />
(Hay-Schmidt 1992). Although it is tempting to speculate that<br />
this neural structure might correspond to the spiralian-type
60 Chapter III<br />
Altenburger and Wanninger<br />
apical organ described herein for Novocrania anomala, it is<br />
important to note (i) that a flask-shaped character could not<br />
be assigned to the apical organ cells of these linguliform paralarvae<br />
and (ii) that the number of cells in their apical organ<br />
is considerably higher than that of the other spiralian larvae.<br />
Overall, the ‘‘apical organ’’ of linguliform larvae resembles<br />
more closely the one found in phoronid and deuterostome<br />
larvae (Santagata 2002), the homology of which remains to be<br />
proven. The suggested derived character of the nervous system<br />
of linguliform brachiopod paralarvae is consistent with<br />
the view that linguliforms have lost the lecithotrophic larva<br />
and have secondarily acquired a planktotrophic life cycle<br />
stage via a stage that resembles a swimming juvenile rather<br />
than a ‘‘true’’ brachiopod larva (Lüter 2001).<br />
We found a-tubulin-positive neural tissue solely in postmetamorphic<br />
specimens of Novocrania anomala. The a-tubulin<br />
signal is located in the same region as the serotonin-like<br />
signal and shows two ventral neurite bundles that are interconnected<br />
by one commissure at the anterior end, one at the<br />
posterior end, and by a median commissure. The fact that we<br />
did not find a-tubulin in the Novocrania anomala larvae that<br />
exhibit serotonergic neurites demonstrates that tubulin<br />
alone is not a reliable marker for nervous structures in<br />
lophotrochozoan larvae. The tubulinergic nervous system in<br />
juvenile Novocrania anomala outlines the adult nervous<br />
system, which consists of two ventral neurite bundles, a subesophageal<br />
and a supraesophageal commissure, and mediodorsal<br />
mantle neurites (Blochmann 1892; Bullock and<br />
Horridge 1965). The anterior ventral neurite bundles form<br />
the arm neurites of the lophophore. Perpendicular from these<br />
arm neurites extend accessory brachial neurites (Williams and<br />
Rowell 1965a, b).<br />
Despite some classical studies, the adult neural anatomy of<br />
brachiopods is only poorly known (James et al. 1992). In the<br />
articulate Gryphus vitreus the nervous system comprises a<br />
transverse supraenteric ganglion and a subenteric ganglion<br />
lying above and below the esophagus, as does the subesophageal<br />
and supraesophageal commissure in Novocrania<br />
anomala (Bullock and Horridge 1965). Our study provides a<br />
first step toward an understanding of the larval anatomy,<br />
neurotransmitter distribution, and development of the nervous<br />
system in brachiopod taxa with lecithotrophic larvae.<br />
Although additional data are needed to assess the brachiopod<br />
neural ground pattern, the finding that serotonergic flaskshaped<br />
cells similar to those found in spiralian larvae do occur<br />
in the apical organ of Novocrania anomala larvae strengthens<br />
the hypo<strong>thesis</strong> that this cell type was also present in the last<br />
common ancestor of Lophotrochozoa (see Wanninger 2009).<br />
Acknowledgments<br />
We are grateful to Matthias Obst and the staff of the Sven Lovén<br />
Centre for Marine Science, Kristineberg, Sweden for help with collection<br />
of adult animals and for providing laboratory space. This<br />
study was funded by the Danish Agency for Science, Technology and<br />
Innovation (grant no. 645-06-0294 to A. W.). Research in the laboratory<br />
of A. W. is further supported by the EU-funded Marie Curie<br />
Network MOLMORPH (contract grant no. MEST-CT-2005-020542).<br />
REFERENCES<br />
Brachiopod neuromuscular development 23<br />
Altenburger, A., and Wanninger, A. 2009. Comparative larval myogenesis<br />
and adult myoanatomy of the rhynchonelliform (articulate) brachiopods<br />
Argyrotheca cordata, A. cistellula, and Terebratalia transversa. Front.<br />
Zool. 6: 3.<br />
Atkins, D., and Rudwick, M. J. S. 1962. The lophophore and ciliary feeding<br />
mechanisms of the brachiopod Crania anomala (Mu¨ ller). J. Mar. Biol.<br />
Assoc. UK 42: 469–480.<br />
Blochmann, F. 1892. Untersuchungen u¨ber den Bau der Brachiopoden. Gustav<br />
Fischer, Jena.<br />
Bulman, O. M. B. 1939. Muscle systems of some inarticulate brachiopods.<br />
Geol. Mag. 76: 434–444.<br />
Bullock, T. H., and Horridge, G. A. 1965. Lophophorate phyla: Ectoprocta,<br />
Brachiopoda, and Phoronida. In Structure and Function in the<br />
Nervous System of Invertebrates. W.H.Freeman,NewYork,pp.631–<br />
647.<br />
Carlson, S. J. 1995. Phylogenetic relationships among extant brachiopods.<br />
Cladistics 11: 131–197.<br />
Cohen, B. L. 2000. Monophyly of brachiopods and phoronids: reconciliation<br />
of molecular evidence with Linnaean classification (the subphylum<br />
Phoroniformea nov.). Proc. R. Soc. B 267: 225–231.<br />
Cohen, B. L., Long, S. L., and Saito, M. 2008. Living craniids: preliminary<br />
molecular evidence of their inter-relationships. Fossils Strata 54: 283–287.<br />
Cohen, B. L., and Weydmann, A. 2005. Molecular evidence that phoronids<br />
are a subtaxon of brachiopods (Brachiopoda: Phoronata) and that genetic<br />
divergence of metazoan phyla began long before the early Cambrian.<br />
Org. Divers. Evol. 5: 253–273.<br />
Chuang, S. H. 1996. Competence, pre- and post-settlement choices of articulate<br />
brachiopod larvae. In P. Copper and J. Jin (eds.). Brachiopods:<br />
Proceedings of the Third International Brachiopod Congress. A.A.Balkema,<br />
Rotterdam, pp. 65–67.<br />
Freeman, G. 1993. Metamorphosis in the brachiopod TerebrataliaFevidence<br />
for a role of calcium-channel function and the dissociation of shell<br />
formation from settlement. Biol. Bull. 184: 15–24.<br />
Freeman, G. 2000. Regional specification during embryogenesis in the<br />
craniiform brachiopod Crania anomala. Dev. Biol. 227: 219–238.<br />
Freeman, G., and Lundelius, J. W. 1999. Changes in the timing of mantle<br />
formation and larval life history traits in linguliform and craniiform<br />
brachiopods. Lethaia 32: 197–216.<br />
Gee, H. 1995. Lophophorates prove likewise variable. Nature 374: 493.<br />
Halanych, K. M. 2004. The new view of animal phylogeny. Annu. Rev. Ecol.<br />
Evol. Syst. 35: 229–256.<br />
Hay-Schmidt, A. 1992. Ultrastructure and immunocytochemistry of the<br />
nervous system of the larvae of Lingula anatina and Glottidia sp. (Brachiopoda).<br />
Zoomorphology 112: 189–205.<br />
Hay-Schmidt, A. 2000. The evolution of the serotonergic nervous system.<br />
Proc. R. Soc. B 267: 1071–1079.<br />
Hejnol, A., et al. 2009. Assessing the root of bilaterian animals with scalable<br />
phylogenomic methods. Proc. R. Soc. B 276: 4261–4270.<br />
Helmcke, J. G. 1939. Die Muskeln der Brachiopoden. Zool. Jahrb. Abt.<br />
Syst. Oekol. Geogr. Tiere 72: 100–140.<br />
Holmer, L. E. 2001. Phylogeny and classification: Linguliformea and<br />
Craniiformea. Paleontol. Soc. Paper 7: 11–26.<br />
James, M. A., Ansell, A. D., Collins, M. J., Curry, G. B., Peck, L. S., and<br />
Rhodes, M. C. 1992. Biology of living brachiopods. Adv. Mar. Biol. 28:<br />
175–387.<br />
Lee, D. E., and Brunton, C. H. C. 1986. Neocrania n. gen., and a revision of<br />
Cretaceous–Recent brachiopod genera in the family Craniidae. Bull. Nat.<br />
Hist. Mus. London (Geol.) 40: 141–160.<br />
Lee, D. E., and Brunton, C. H. C. 2001. Novocrania, anewnameforthe<br />
genus Neocrania Lee & Brunton, 1986 (Brachiopoda, Craniida), preoc-
Chapter III<br />
61<br />
24 EVOLUTION & DEVELOPMENT Vol. 12, No. 1, January--February 2010<br />
cupied by Neocrania Davis, 1978 (Insecta, Lepidoptera). Bull. Nat. Hist.<br />
Mus. London (Geol.) 57: 5.<br />
Lu¨ ter, C. 2001. Brachiopod larval setaeFa key to the phylum’s ancestral<br />
life cycle? In C. H. C. Brunton, L. R. M. Cocks, and S. L. Long (eds.).<br />
Brachiopods Past and Present. Taylor & Francis, London, pp. 46–55.<br />
Nielsen, C. 1991. The development of the brachiopod Crania (Neocrania)<br />
anomala (O. F. Mu¨ ller) and its phylogenetic significance. Acta Zool. 72:<br />
7–28.<br />
Nielsen, C. 2002. The phylogenetic position of Entoprocta, Ectoprocta,<br />
Phoronida, and Brachiopoda. Integr. Comp. Biol. 42: 685–691.<br />
Paps, J., Bagun˜ a` , J., and Riutort, M. 2009. Lophotrochozoa internal phylogeny:<br />
new insights from an up-to-date analysis of nuclear ribosomal<br />
genes. Proc. R. Soc. B 276: 1245–1254.<br />
Peck, L. S., and Robinson, K. 1994. Pelagic larval development in<br />
the brooding Antarctic brachiopod Liothyrella uva. Mar. Biol. 120: 279–<br />
286.<br />
Pires, A., and Woollacott, R. M. 1997. Serotonin and dopamine have opposite<br />
effects on phototaxis in larvae of the bryozoan Bugula neritina.<br />
Biol. Bull. 192: 399–409.<br />
Rowell, A. J. 1960. Some early stages in the development of the brachiopod<br />
Crania anomala (Mu¨ ller). Ann. Mag. Nat. Hist. 13: 35–56.<br />
Sakarya, O., et al. 2007. A post-synaptic scaffold at the origin of the animal<br />
kingdom. PLoS One 2: e506.<br />
Santagata, S. 2002. Structure and metamorphic remodeling of the larval<br />
nervous system and musculature of Phoronis pallida (Phoronida). Evol.<br />
Dev. 4: 28–42.<br />
Shimizu, K., Hunter, E., and Fusetani, N. 2000. Localisation of biogenic<br />
amines in larvae of Bugula neritina (Bryozoa: Cheilostomatida) and their<br />
effects on settlement. Mar. Biol. 136: 1–9.<br />
Stricker, S. A., and Reed, C. G. 1985. The ontogeny of shell secretion in<br />
Terebratalia transversa (Brachiopoda, Articulata). 1. Development of the<br />
mantle. J. Morphol. 183: 233–250.<br />
Voronezhskaya, E. E., Tsitrin, E. B., and Nezlin, L. P. 2003. Neuronal<br />
development in larval polychaete Phyllodoce maculata (Phyllodocidae).<br />
J. Comp. Neurol. 455: 299–309.<br />
Voronezhskaya, E. E., Tyurin, S. A., and Nezlin, L. P. 2002. Neuronal<br />
development in larval chiton Ischnochiton hakodadensis (Mollusca: Polyplacophora).<br />
J. Comp. Neurol. 444: 25–38.<br />
Wanninger, A. 2009. Shaping the things to come: ontogeny of Lophotrochozoan<br />
neuromuscular systems and the Tetraneuralia concept. Biol.<br />
Bull. 216: 293–306.<br />
Wanninger, A., Fuchs, J., and Haszprunar, G. 2007. Anatomy of the<br />
serotonergic nervous system of an entoproct creeping-type larva and its<br />
phylogenetic implications. Invertebr. Biol. 126: 268–278.<br />
Wanninger, A., and Haszprunar, G. 2003. The development of the<br />
serotonergic and FMRF-amidergic nervous system in Antalis entalis<br />
(Mollusca, Scaphopoda). Zoomorphology 122: 77–85.<br />
Williams, A., Carlson, S. J., Brunton, C. H. C., Holmer, L. E., and Popov,<br />
L. 1996. A supra-ordinal classification of the Brachiopoda. Proc. R. Soc.<br />
B 351: 1171–1193.<br />
Williams, A., and Rowell, A. J. 1965a. Brachiopod anatomy. In R. C.<br />
Moore (ed.). Treatise on Invertebrate Paleontology, Part H, Brachiopoda.<br />
Geological Society of America and University of Kansas Press, Lawrence,<br />
KS, pp. 6–57.<br />
Williams, A., and Rowell, A. J. 1965b. Evolution and phylogeny. In R. C.<br />
Moore (ed.). Treatise on Invertebrate Paleontology, Part H, Brachiopoda.<br />
The Geological Society of America and The University of Kansas Press,<br />
Lawrence, KS, pp. 164–199.
62 Chapter IV<br />
Chapter IV<br />
Altenburger, A., Martinez, P. & Wanninger, A. First expression<br />
study of homeobox genes in Brachiopoda: the role of Not and Cdx<br />
in bodyplan patterning and germ layer specification. Submitted
Chapter IV<br />
Submitted manuscript<br />
63<br />
First expression study of homeobox genes<br />
in Brachiopoda: the role of Not and Cdx in<br />
bodyplan patterning, neurogenesis, and germ<br />
layer specification<br />
Andreas Altenburger 1 , Pedro Martinez 2, 3, Andreas Wanninger 1*<br />
1<br />
University of Copenhagen, Department of Biology, Research Group for Comparative Zoology,<br />
Universitetsparken 15, DK-2100 Copenhagen Ø, Denmark<br />
2<br />
Universitat de Barcelona, Facultat de Biología, Departament de Genètica, Av. Diagonal 645,<br />
ES-08028 Barcelona, Spain<br />
3<br />
Institució Catalana de Recerca i Estudis Avançats (ICREA)<br />
*corresponding author. E-mail: awanninger@bio.ku.dk<br />
ABSTRACT<br />
Not is a homeobox containing gene<br />
that regulates the formation of the<br />
notochord in chordates, while Caudal<br />
(Cdx) is a ParaHox gene involved in<br />
the formation of posterior tissues of<br />
various animal phyla. Here, we present<br />
the first expression data of a Not<br />
and a Cdx homolog in the articulate<br />
brachiopod Terebratalia transversa.<br />
The T. transversa homolog, TtrNot,<br />
is expressed in the ectoderm from<br />
the beginning of gastrulation until<br />
completion of larval development,<br />
which is marked by a three-lobed body<br />
with larval setae. Expression starts at<br />
gastrulation in two areas lateral to the<br />
blastopore and subsequently extends<br />
over the animal pole of the gastrula.<br />
With elongation of the gastrula,<br />
expression at the animal pole narrows<br />
to a small band, whereas the areas<br />
lateral to the blastopore shift slightly<br />
towards the future anterior region of<br />
the larva. Upon formation of the three<br />
larval body lobes, TtrNot expressing<br />
cells are present only in the posterior<br />
part of the apical lobe. Expression<br />
ceases entirely at the onset of larval<br />
setae formation. TtrNot expression<br />
is absent in unfertilized eggs, in<br />
INTRODUCTION<br />
Homeobox genes are characterized<br />
by the presence of a short, wellconserved<br />
DNA fragment, which<br />
encodes for the homeodomain. The<br />
latter is a protein motif of 60 to 63<br />
amino acids, which was first described<br />
embryos prior to gastrulation, and in<br />
settled individuals during and after<br />
metamorphosis. Comparison with<br />
the expression patterns of Not genes<br />
in other metazoan phyla suggests<br />
an ancestral role in gastrulation and<br />
germ layer (ectoderm) specification<br />
with co-opted functions in notochord<br />
formation in chordates and left/right<br />
determination in ambulacrarians and<br />
vertebrates. TtrCdx is first expressed<br />
after gastrulation in the ectoderm of the<br />
gastrula in the posterior region of the<br />
blastopore. Its expression stays stable<br />
in the ectoderm at the posterior pole<br />
of the blastopore until the blastopore<br />
is closed. Thereafter, the expression<br />
remains in the ventral portion of the<br />
mantle lobe of the fully developed larva.<br />
No TtrCdx expression is detectable in<br />
the juvenile after metamorphosis. The<br />
expression of TtrCdx is congruent with<br />
findings in other metazoans, were<br />
genes belonging to the Cdx/caudal<br />
family are predominantly localized<br />
posteriorly during gastrulation and<br />
subsequently play a role in the<br />
formation of posterior tissues.<br />
for Drosophila melanogaster homeotic<br />
genes, and subsequently was found<br />
in all animal phyla studied to date<br />
(McGinnis et al. 1984, Scott and<br />
Weiner 1984, Lanfear and Bromham<br />
2008). Homeobox genes function as<br />
developmental control genes that
64 Submitted manuscript<br />
Chapter IV<br />
encode transcription factors which<br />
activate gene cascades (Hueber and<br />
Lohmann 2008). In the case of the<br />
Not gene, which plays an important<br />
role during notochord formation in<br />
vertebrates (Stein and Kessel 1995,<br />
Talbot et al. 1995, Gont et al. 1996, Stein<br />
et al. 1996, Abdelkhalek et al. 2004),<br />
the downstream genes are known to<br />
regulate mesoderm formation in sea<br />
urchins as well as left/right patterning,<br />
notochord, mesoderm, and somite<br />
formation in vertebrates (Peterson et<br />
al. 1999, Yasuo and Lemaire 2001,<br />
Beckers et al. 2007). Homologs of<br />
the homeobox gene Not have been,<br />
among others, identified in Xenopus<br />
(Xnot), chick (Gnot1, Gnot2), zebrafish<br />
(flh), mouse (noto), Hydra (HvuNot),<br />
Drosophila (90Bre), and the basal<br />
eumetazoan Trichoplax adhaerens<br />
(TadNot), but the developmental<br />
role of Not in invertebrates without a<br />
notochord is largely unknown (Dessain<br />
and McGinnis 1993, von Dassow et al.<br />
1993, Knezevic et al. 1995, Odenthal<br />
et al. 1996, Gauchat et al. 2000,<br />
Martinelli and Spring 2004, Hoskins<br />
et al. 2007). A Not homolog seems<br />
to be lacking in the model sponge<br />
Amphimedon queenslandica (Bernard<br />
Degnan, personal communication).<br />
However, the presence of a Not gene<br />
in cnidarians and Trichoplax indicates<br />
that it was present prior to the evolution<br />
of the mesoderm, and thus long<br />
before the evolution of the notochord.<br />
Accordingly, the ancestral role of Not<br />
remains elusive. Given its confirmed<br />
absence in the poriferan genome<br />
would make it a good candidate for a<br />
eumetazoan apomorphy.<br />
Cdx is a member of the ParaHox gene<br />
cluster which probably originated by<br />
duplication from an ancestral ProtoHox<br />
gene cluster which led to the Hox and<br />
ParaHox clusters, respectively (Brooke<br />
et al. 1998). Cdx has been found to be<br />
involved in the development of posterior<br />
tissues of almost all animal phyla in<br />
which it has been investigated and is<br />
thus often termed “caudal” (Epstein et<br />
al. 1997, Copf et al. 2004). In addition<br />
to the posterior tissues, it was found<br />
to be expressed in the mesoderm of<br />
taxa as diverse as Artemia, Capitella,<br />
Patella, Branchiostoma, and Mus;<br />
in the gut of Drosophila, Capitella,<br />
Branchiostoma, and Mus; and in the<br />
central nervous system of Capitella,<br />
Branchiostoma, and Mus (Macdonald<br />
and Struhl 1986, Duprey et al. 1988,<br />
Le Gouar et al. 2003, Copf et al.<br />
2004, Fröbius and Seaver 2006). Cdx<br />
is absent in the recently sequenced<br />
poriferan Amphimedon queenslandica<br />
(Larroux et al. 2008, Srivastava et<br />
al. 2010), but a gene related to Cdx<br />
is present in Nematostella vectensis,<br />
a representative of Cnidaria, the<br />
proposed sister group to Bilateria<br />
(Chourrout et al. 2006, Quiquand et<br />
al. 2009).<br />
The phylogenetic position of<br />
Brachiopoda within Bilateria is still<br />
controversial (Williams and Carlson<br />
2007, Hejnol et al. 2009, Paps et al.<br />
2009). Most authors include them within<br />
Lophotrochozoa, but their position<br />
within this clade remains unresolved,<br />
and some authors consider them a<br />
sister group to Deuterostomia (Nielsen<br />
2002). Within the phylum, Brachiopoda<br />
comprises three clades: Linguliformea,<br />
Craniiformea, and Rhynchonelliformea.<br />
Linguliformea and Craniiformea are<br />
often considered sister groups and<br />
were traditionally termed “inarticulate”,
Chapter IV<br />
Submitted manuscript<br />
65<br />
because their valves are not connected<br />
to each other by a hinge (Cohen and<br />
Weydmann 2005). We investigated the<br />
brachiopod Terebratalia transversa, a<br />
representative of the rhynchonelliform<br />
(articulate) brachiopods, the largest<br />
group within recent Brachiopoda. So<br />
far, several Hox gene sequences have<br />
been characterized for the linguliform<br />
brachiopod Lingula anatina (de Rosa et<br />
al. 1999). However, no expression data<br />
for any Hox or homeobox containing<br />
genes are currently available for<br />
Brachiopoda. With the investigation of<br />
Not and Cdx expression in Terebratalia<br />
transversa we aim to shed light on the<br />
function of these genes in invertebrate<br />
body patterning and thereby contribute<br />
to the discussion concerning their<br />
ancestral roles in eumetazoan (i.e.,<br />
placozoan, diploblast, and triploblast)<br />
development and evolution.<br />
MATERIAL AND METHODS<br />
Animal collection, rearing, and<br />
fixation<br />
Adult animals were dredged in the vicinity<br />
of the Friday Harbor Laboratories,<br />
Washington, USA, at 48º32’869 N;<br />
122º58’452 W during summer 2008<br />
and spring 2009. The animals were<br />
placed in running seawater tables<br />
at ambient seawater temperature<br />
(approx. 11.5ºC). Embryos were<br />
obtained by artificial fertilization. To<br />
this end, gonads were dissected from<br />
the specimens and stored individually<br />
in beaker glasses. The eggs were<br />
washed several times with seawater<br />
and left in 100ml seawater until<br />
germinal vesicle breakdown, which<br />
usually occurred within 10-16 hours<br />
after dissection. Sperm cells were left<br />
until they had acquired a high degree<br />
of motility, which usually occurred after<br />
4-14 hours. Sperm remained active<br />
until up to 48 hours after dissection.<br />
For fertilization, a few drops of the<br />
sperm suspension were added to the<br />
beaker glasses containing the eggs.<br />
Development of embryos and larvae<br />
was monitored closely and the beaker<br />
glasses were cleaned daily from debris<br />
with help of a glass pipette driven by a<br />
peristaltic pump. Larvae were fixed at<br />
various developmental stages in 4%<br />
paraformaldehyde in 0.5M NaCl, 0.1M<br />
MOPS (pH 7.5) for 8-10 hours at 4ºC,<br />
washed in 50% EtOH for 30 min, and<br />
finally stored in 80% EtOH at -20ºC.<br />
Cloning and in situ hybridization<br />
RNA was extracted from larvae<br />
at various developmental stages<br />
with a miRCURY RNA Isolation Kit<br />
(Exiqon, Vedbaek, Denmark). It was<br />
reversely transcribed into cDNA with<br />
a RETROscript Kit using oligo(dT)<br />
primers (Applied Biosystems/Ambion,<br />
Austin, TX, USA). In order to screen<br />
for homeobox containing genes, the<br />
cDNA was used as template for PCR<br />
reactions with the following degenerate<br />
primers: HoxF 5’-GCT CTA GAR YTN<br />
GAR AAR GAR TT-3’, which recognizes<br />
the peptide sequence ELEKEF, and<br />
HoxR 5’-GGA ATT CRT TYT GRA<br />
ACC ADA TYT T-3’, which recognizes<br />
the peptide sequence KIWFQN<br />
(Murtha et al. 1991; Balavoine and<br />
Telford 1995). PCR was carried out<br />
under the following conditions: 3 min<br />
94 °C, followed by 40 cycles of 45s at<br />
94 °C, 45s at 50 °C, and 60s at 72 °C,<br />
followed by a final extension step of<br />
10 min at 72 °C. PCR products were<br />
purified over column with a QIAquick<br />
Gel Extraction Kit (Qiagen, Venlo, The
66 Submitted manuscript<br />
Chapter IV<br />
Netherlands) and subsequently ligated<br />
into a pGEM-T Easy vector (Promega,<br />
Madison, WI, USA). Ligation products<br />
were transformed into One Shot<br />
TOP10 E. coli competent cells<br />
(Invitrogen, Carlsbad, CA, USA). Cells<br />
were allowed to grow over night; clone<br />
DNA was isolated using a QIAprep<br />
Spin Miniprep Kit (Qiagen). Insert<br />
sequences were sequenced at the<br />
sequencing facility of the University<br />
of Barcelona and identified using<br />
the tBLASTx algorithm. Two specific<br />
forward primers were subsequently<br />
designed from the TtrNot and the<br />
TtrCdx PCR sequences: TtrNotF1 5’-<br />
GGA GAA GGA GTT CGA AAG GCA<br />
ACA A-3’, TtrNotF2 5’-CCG AAT CCC<br />
AAG TGA AGA TCT GGT-3’, TtrCdxF1<br />
5’-CCT GGA GCT GGA GAA GGA<br />
GTT CTG T-3’, and TtrCdxF2 5’-AAC<br />
AAC CTT GTA CTT TCA GAG AGA<br />
CAG G-3’. The specific primers were<br />
used nested in a 3’RACE-PCR using<br />
a SMART RACE kit following the<br />
manufacturer’s protocol (Clontech,<br />
Mountain View, CA, USA). The<br />
sequences of the RACE-PCR products<br />
were again checked by BLAST and the<br />
positive clones were used for in situ<br />
probe production using the DIG RNA<br />
Labeling Kit (SP6/T7, Roche, Basel,<br />
Switzerland).<br />
In situs were done following a standard<br />
protocol with a 5 min proteinase K step<br />
and at least 48 hours of hybridization<br />
time at 40ºC or 45ºC (Martindale<br />
et al. 2004; Hejnol and Martindale<br />
2008). For cohorts aged 0-64 hours<br />
after fertilization (hpf), in situs were<br />
performed on developmental stages<br />
that were 2-4 hours apart, for the age<br />
group of 64-154 hpf, in situs were<br />
done every 5-10 hours, while for later<br />
stages longer intervals were chosen.<br />
The latest stages investigated were<br />
540 hpf old, which corresponded to<br />
420 hours after settlement/onset of<br />
metamorphosis (hps). Sense probes<br />
were generated as controls for in situ<br />
hybridization. Since they didn’t give<br />
any signal they are omitted in the<br />
figures.<br />
Stained specimens were photographed<br />
with a Leica ProgRes C3 digital<br />
camera mounted on a Leica MZ<br />
16F stereomicroscope. Schematic<br />
illustrations were generated using<br />
Adobe Illustrator CS3 and CS4<br />
graphics software (Adobe, San Jose,<br />
CA, USA). Analysis of gene sequences<br />
and primer design was done with CLC<br />
Main Workbench 5 (CLC bio, Aarhus,<br />
Denmark).<br />
Immunostaining and confocal<br />
laserscanning microscopy<br />
Larvae were stained with antibodies<br />
against serotonin (ImmunoStar,<br />
Hudson, WI, USA) and acetylated<br />
α-tubulin (Sigma-Aldrich, St. Louis,<br />
MO, USA). In addition, cell nuclei<br />
were labeled using DAPI (Invitrogen,<br />
Eugene, OR, USA). Prior to staining,<br />
larvae were washed thrice for 15min<br />
each in phosphate buffer (PB) and<br />
incubated for 1h in PB containing<br />
0.2% Triton X-100 (Sigma-Aldrich)<br />
at room temperature. Thereafter,<br />
the larvae were incubated over night<br />
at 4ºC in 6% normal goat serum<br />
in 0.1M PB and 0.2% Triton X-100<br />
(blocking solution). Then, the larvae<br />
were incubated for 24 hours at 4ºC in<br />
blocking solution containing a 1:800<br />
dilution of the polyclonal serotonin<br />
antibody, 3µg/ml DAPI, and a 1:800<br />
dilution of the monoclonal acetylated
Chapter IV<br />
Submitted manuscript<br />
67<br />
Fig. 1 Characterization of the Not sequence of Terebratalia transversa. (A) Not<br />
homeodomain sequence alignment. The accession numbers for the EMBL/GenBank databases<br />
are given in brackets: Terebratalia transversa (Ttr, brachiopod, XXXXXXX), Nematostella<br />
vectensis (Nve, cnidarian, XP_001641364.1), Hydra vulgaris (Hvu, cnidarian, CAB88387.1),<br />
Trichoplax adhaerens (Tad, placozoan, AAQ82694.1), Drosophila melanogaster (Dme, fruit fly,<br />
NP_650701.1), Strongylocentrotus purpuratus (Spu, sea urchin, AAD20328.1), Hemicentrotus<br />
pulcherrimus (Hpu, sea urchin, BAD91047.1), Branchiostoma floridae (Bfl, Florida lancelet,<br />
XP_002601133.1), Danio rerio (Dre, zebrafish, NP_571130.1), Xenopus laevis (X, frog,<br />
NP_001081625.1). The following alternative species and Hox protein sequences were chosen<br />
as outgroups: Drosophila virilis, Antennapedia (DviAnt, fruit fly, AAQ67266.1), Drosophila<br />
melanogaster, Proboscipedia (DmePb, fruit fly, CAA45272), Neanthes virens, Hox7 and<br />
Engrailed (NviHox7 and NviEng, annelid, DQ366682 and DQ366680). Dots represent amino<br />
acid identity with the amino acid sequence of the T. transversa Not protein shown at the top of<br />
the alignment. (B) Alignment tree based on the 52 amino acid sequences shaded in Fig. 1A.<br />
Algorithm = UPGMA; Bootstrap = 10.000 replicates. Bootstrap values are given for each node.<br />
Due to the small number of residues for the analysis, the phylogenetic signal of the tree is<br />
limited. The tree shows, however, that TtrNot clusters with all other Not protein sequences and<br />
thus is a true Not protein.<br />
α-tubulin antibody. Subsequently,<br />
the larvae were washed four times<br />
over a period of 12h in PB containing<br />
0.2% Triton X-100 and an Alexa Fluor<br />
633-conjugated goat anti-rabbit as<br />
well as an Alexa Fluor 488-conjugated<br />
goat anti-mouse secondary antibody<br />
(Invitrogen) in a dilution of 1:400<br />
for 24h at 4ºC. Finally, the larvae<br />
were washed tree times for 15 min<br />
each in 0.1M PB and embedded in<br />
Flouromount G (Southern Biotech,<br />
Birmingham, AL, USA) on glass<br />
slides. The samples were analyzed<br />
with a Leica TCS SP5 II confocal<br />
system (Leica Microsystems, Wetzlar,<br />
Germany). The resulting image stacks<br />
were merged into maximum projection<br />
images and assembled using Adobe<br />
Photoshop CS3 software (Adobe, San<br />
Jose, CA, USA).
68 Submitted manuscript<br />
Chapter IV<br />
Fig. 2 Characterisation of the Cdx sequence of Terebratalia transversa. (A) Cdx<br />
homeodomain sequence alignment. The accession numbers for the EMBL/GenBank databases<br />
are given in brackets: Terebratalia transversa (Ttr, brachiopod, XXXXXXX), Nematostella<br />
vectensis (Nve, cnidarian, DQ500749), Patella vulgata (Pvu, gastropod, AJ518062.1), Capitella<br />
teleta (Cte, annelid, AAZ95508.1), Drosophila melanogaster (Dme, fruit fly, NM_057606.4),<br />
Tribolium castaneum (Tca, flour beetle, NM_001039409.1), Ciona intestinalis (Cin, tunicate,<br />
NP_001071669), Saccoglossus kowalevskii (Sko, hemichordate, NP_001158415), and Mus<br />
musculus (Mmu, mouse, NM_009880.3). The following alternative species and Hox protein<br />
sequences were chosen as outgroups: Drosophila simulans, Abdominal B (DsiAbdB, fruit<br />
fly, XP_002103136), Drosophila melanogaster, Proboscipedia (DmePb, fruit fly, CAA45272),<br />
Drosophila virilis, Antennapedia (DviAnt, fruit fly, AAQ67266.1), Mus musculus, HoxA9<br />
(MmuHoxA9, mouse, NP_034586.1), Neanthes virens, Engrailed (NviEng, annelid, DQ366680).<br />
Dots represent amino acid identity with the amino acid sequence of the T. transversa Cdx<br />
protein shown at the top of the alignment. (B) Alignment tree based on the 49 amino acid<br />
sequences is shaded in Fig. 2A. Algorithm = UPGMA; Bootstrap = 10.000 replicates. Bootstrap<br />
values are given for each node. Due to the small number of residues used in the analysis, the<br />
phylogenetic signal of the tree is limited. The tree shows, however, that TtrCdx clusters with all<br />
other Cdx/caudal protein sequences and thus is a true Cdx protein.<br />
RESULTS<br />
Characterization of the Terebratalia<br />
Not and Cdx genes<br />
The PCR-amplified region of the TtrNot<br />
homeobox encodes for a 45 amino<br />
acids long peptide which is largely<br />
similar to the NveNot sequence of the<br />
cnidarian Nematostella vectensis (82%<br />
sequence identity). This peptide has<br />
clear affinities to the HvuNot protein<br />
of the cnidarian Hydra vulgaris (69%<br />
sequence identity, Fig. 1). The TtrNot<br />
segment cloned by RACE-PCR was<br />
667 base pairs long and is deposited<br />
in GenBank under the accession<br />
number XXXXXX. The transcribed<br />
region downstream of the homeobox<br />
ends with a poly-A stretch and shows<br />
no similarity to other known gene<br />
sequences.<br />
The conserved protein sequence of the<br />
TtrCdx homeobox identified in this study
Chapter IV<br />
Submitted manuscript<br />
69<br />
Fig. 3 Not expression in Terebratalia transversa. Scale bars equal 50 µm, age of specimens<br />
is given in hours after fertilization (hpf) or hours after settlement (hps), respectively. Blue<br />
represents areas of TtrNot expression. (A) Fertilized egg lacking TtrNot expression. (B) 16<br />
cell stage. (C) 32-64 cell stage. (D) Blastula at the onset of gastrulation. TtrNot is expressed<br />
in two fields of cells (arrows) lateral to the future blastopore. (E) Early gastrula in vegetal view<br />
showing the blastopore (asterisk) and two fields of TtrNot expressing cells. (F) Same stage<br />
as in E, lateral view, the blastopore is on the lower side (asterisk). The lateral fields of TtrNot<br />
extend into the animal pole of the gastrula. The ectoderm (ec) and endoderm (en) have started<br />
to form and are demarcated by a dashed line for clarity. (G) The TtrNot expressing cells extend<br />
in a horseshoe-like pattern over the entire gastrula. (H) Lateral view of a late gastrula with<br />
widened archenteron. The TtrNot expressing cells extend over the entire ectoderm (ec) lateral<br />
to the blastopore (asterisk). The lateral fields of TtrNot expressing cells are interconnected via<br />
a small band of TtrNot expressing cells (arrowhead) on the animal pole of the gastrula. TtrNot is<br />
not expressed in the endoderm (en). (I) Slightly elongated gastrula stage, vegetal view showing<br />
the blastopore (asterisk). The position of TtrNot expressing cells has slightly shifted towards<br />
the animal pole, i.e., the future anterior region of the larva. (J) Further elongated gastrula with<br />
blastopore (asterisk) and two fields of TtrNot expressing cells, which are interconnected by a<br />
narrow band of TtrNot expressing cells (arrowhead) in the animal region of the gastrula. (K)
70 Submitted manuscript<br />
Chapter IV<br />
Same stage as in J with animal view onto the future dorso-anterior part of the larva. The two<br />
fields of TtrNot expressing cells which are interconnected by a narrow band of TtrNot expressing<br />
cells (arrowhead) are visible. (L) Elongated gastrula with small blastopore (asterisk). The TtrNot<br />
expressing cells are distributed equally along the posterior part of the future larval apical lobe.<br />
(M) Ventral view of an early three-lobed larva with apical tuft (at), anterior lobe (al), mantle lobe<br />
(ml), and pedicle lobe (pl). The blastopore (asterisk) is closed. The border between ectoderm<br />
(ec) and mesoderm (ms) is visible in the mantle and pedicle lobe. TtrNot expressing cells are<br />
distributed in the posterior part of the apical lobe (indicated by the dashed line). (N) Ventroanterior<br />
view of a larva with all larval lobes fully established: apical lobe (al), mantle lobe (ml),<br />
pedicle lobe (pl). The blastopore (asterisk) is closed and TtrNot expressing cells are distributed<br />
in a ring along the posterior part of the apical lobe. (O) Dorsal view of a fully differentiated<br />
larva with apical lobe (al), mantle lobe (ml), pedicle lobe (pl), and setae (s). TtrNot is no longer<br />
expressed. (P) Posterior view of a juvenile at 360 hours after settlement (hps) with pedicle (p),<br />
anlage of both valves (v) ,and larval setae (s), which extend beyond the valves. TtrNot is not<br />
expressed.<br />
Fig. 4 Cdx expression in<br />
Terebratalia transversa. Scale bars<br />
equal 50 µm, age is given in hours<br />
after fertilization (hpf). (A) Gastrula<br />
stage, TtrCdx (purple) is expressed in<br />
the ectoderm of the posterior pole of<br />
the blastopore (asterisk). (B) Gastrula<br />
stage slightly older than the one in A,<br />
TtrCdx expression on the posterior<br />
side of the blastopore (asterisk) is<br />
more intense than in A. (C) Maximum<br />
projection image of a confocal<br />
reflection scan of a specimen with<br />
similar expression pattern as in B,<br />
showing that TtrCdx is still limited to<br />
the ectoderm. (D) Early larva with<br />
modest expression of TtrCdx (arrow)<br />
in the ventral part of the future mantle<br />
lobe (ml), immediately posterior to<br />
the blastopore (asterisk). The future<br />
apical lobe (al) and posterior lobe (pl)<br />
can already be distinguished. (E) Early<br />
three-lobed larva with apical lobe (al),<br />
mantle lobe (ml), and pedicle lobe<br />
(pl). TtrCdx (arrow) is expressed in<br />
the posterior part of the mantle lobe,<br />
further posterior from the almost closed blastopore (asterisk) than in previous stages. The<br />
larval apical lobe (al), mantle lobe (ml), and pedicle lobe (pl) are further developed. (F) Fully<br />
established larva with expression of TtrCdx (arrow) in the center of the ventral part of the mantle<br />
lobe (ml). Apical lobe (al), mantle lobe, and pedicle lobe (pl) are fully developed and four sets<br />
of larval setae (se) extend from the mantle lobe.
Chapter IV<br />
Submitted manuscript<br />
71<br />
shows 73% -80% sequence similarity<br />
with Cdx/caudal protein sequences<br />
known from other metazoans and 61%<br />
sequence similarity with the Cdx/Xlox<br />
sequence of Nematostella vectensis<br />
(Fig. 2). The TtrCdx segment cloned<br />
by RACE PCR was 1037 base pairs<br />
long and is deposited in GeneBank<br />
under the accession number XXXX.<br />
The transcribed region downstream of<br />
the homeobox ends with a poly-A tail<br />
and shows no similarity to other known<br />
gene sequences.<br />
Not and Cdx expression during<br />
Terebratalia development<br />
Cleavage in Terebratalia transversa<br />
is radial. Within eight hours after<br />
fertilization (hpf), the embryo develops<br />
into a blastula. Gastrulation starts at 18<br />
hpf. The spherical gastrula has a central<br />
blastopore until approximately 26 hpf.<br />
Thereafter, the gastrula elongates and<br />
the blastopore narrows to a slit, moves<br />
anteriorly, and closes at around 42 hpf.<br />
At this stage, the elongated larva starts<br />
to develop its characteristic three–<br />
lobed body, consisting of an anterior<br />
lobe, a mantle lobe, and a pedicle<br />
lobe. At around 65 hpf, the larval setae<br />
start to form and larval development is<br />
complete by approximately 80-96 hpf.<br />
Under our experimental conditions,<br />
larvae settled between 120 and 240<br />
hpf. The expression patterns of TtrNot<br />
and TtrCdx are shown in Figs. 3–5.<br />
Fertilized eggs and early cleavage<br />
stages did not reveal the presence of<br />
a TtrNot RNA transcript (Fig. 3A-C).<br />
Expression of TtrNot starts laterally<br />
on both sides of the blastopore in<br />
the early gastrula stage at 18 hpf<br />
(Fig. 3D). At 22 hpf, the expression<br />
of TtrNot increases in the ectoderm<br />
on both sides of the blastopore and<br />
extends towards the animal pole of the<br />
gastrula (Figs. 3E, F, 5A). These lateral<br />
bands of TtrNot expressing cells fuse<br />
in slightly older stages (i.e., at 24 hpf;<br />
Fig. 3G). Shortly after that, at 26 hpf,<br />
when the archenteron is enlarged, the<br />
band of TtrNot expressing cells on the<br />
animal pole narrows (Figs. 3H, 5B). At<br />
28 hpf the blastopore starts to become<br />
slit-like, the gastrula elongates, and<br />
TtrNot expressing cells are present in<br />
two fields lateral of the blastopore and<br />
close to the future anterior pole of the<br />
larva. The fields of TtrNot expressing<br />
cells are interconnected by a narrow<br />
band of TtrNot expressing cells on the<br />
animal side (Figs. 3I-K, 5C). At around<br />
36 hpf the blastopore starts to close,<br />
the larva elongates further, and TtrNot<br />
expressing cells are only detected<br />
in the animal region of the gastrula,<br />
which later forms the larval apical lobe<br />
(Fig. 3L). Subsequently, the blastopore<br />
closes completely and the larva<br />
differentiates the three characteristic<br />
body lobes. TtrNot is continuously<br />
expressed in a ring of cells in the<br />
apical lobe until the beginning of setae<br />
formation. The area of the apical lobe<br />
where TtrNot is expressed corresponds<br />
to the region that bears the cilia used<br />
for swimming of the larva (Figs. 3M,<br />
N, 5D). Once the larval lobes are fully<br />
established and setae formation has<br />
started (i.e., at around 75 hpf), TtrNot<br />
is no longer detectable (Figs. 3O, 5E).<br />
Likewise, specimens that have settled<br />
and started to metamorphose do not<br />
show any TtrNot expression (Figs. 3P,<br />
5F).<br />
TtrCdx starts to be expressed in<br />
the ectoderm of the gastrula at the<br />
posterior pole of the blastopore (Figs.
72 Submitted manuscript<br />
Chapter IV<br />
Fig. 5 Schematic representation of Not and Cdx expression in Terebratalia transversa.<br />
Dark grey – ectoderm, light grey – endoderm. All specimens are approximately 120 µm in<br />
diameter. (A) Almost spherical gastrula at 22 hours after fertilization (hpf). TtrNot is expressed<br />
in two lateral fields of the ectoderm. TtrCdx is expressed on the posterior side of the blastopore.<br />
(B) Gastrula at 26 hpf. The two lateral fields of TtrNot expressing cells are interconnected by<br />
a narrow band of TtrNot expressing cells on the animal side of the gastrula. TtrCdx is more<br />
intensely expressed posterior to the blastopore. (C) Early larva at 30 hpf. The fields of TtrNot<br />
expressing cells are positioned further towards the animal pole of the gastrula than in previous<br />
stages. The blastopore is still open. TtrCdx is expressed posterior to the blastopore. (D) Larva<br />
at the onset of lobe formation at 42 hpf. TtrNot is solely expressed in the posterior part of the<br />
apical lobe. Locomotory cilia and an apical tuft are already present. TtrCdx is expressed in the<br />
postero-ventral part of the mantle lobe. (E) Three-lobed larva at 75 hpf. TtrNot is no longer<br />
expressed but TtrCdx is still present in the posterior part of the mantle lobe. (F) Juvenile at<br />
360 hours after settlement. The lophophore has started to develop between the valves. Neither<br />
TtrNot nor TtrCdx are expressed.<br />
4A, 5A). TtrCdx expression remains<br />
in this position in the larval ectoderm<br />
throughout development. Expression<br />
intensifies as the gastrula gets older<br />
(Figs. 4B, C, 5B). In early larvae prior<br />
to the onset of lobe formation (Figs.<br />
4D, 5C), early three-lobed larvae (Figs.<br />
4E, 5D), and fully developed larvae<br />
(Figs. 4F, 5E) TtrCdx is expressed in<br />
the ventral ectoderm posterior to the<br />
closed blastopore.<br />
Neither the expression of TtrNot nor<br />
the expression of TtrCdx is co-located<br />
with the larval serotonergic nervous<br />
system of Terebratalia transversa,<br />
which comprises an apical organ with<br />
eight flask-shaped serotonergic cells<br />
that lie antero-dorsally in the apical<br />
lobe (Fig. 5). The flask-shaped cells<br />
are connected via individual neurites<br />
to a larval neuropil that is situated in<br />
the center of the apical lobe. (Fig. 5A,
Chapter IV<br />
Submitted manuscript<br />
73<br />
Fig. 6 Larval<br />
serotonergic nervous<br />
system of Terebratalia<br />
transversa.<br />
Maximum projections of<br />
a confocal microscopy<br />
image stack. Serotonin<br />
is labeled red, tubulin<br />
is green, cell nuclei are<br />
blue. (A) Lateral view<br />
of a fully established<br />
larva with apical lobe<br />
(al), mantle lobe (ml), and pedicle lobe (pl). The apical organ comprises eight flask-shaped<br />
cells (arrows) which are connected by neurites (empty arrowheads) to a larval anterior neuropil<br />
(asterisk). The apical organ is situated towards the dorsal side of the apical lobe. Note that<br />
only cilia but no neural structures are labeled by the α-tubulin antibody. (B) Detailed view of<br />
the apical organ of the specimen shown in A. The apical organ comprises two sets of four<br />
flask-shaped cells each (arrowheads). The flask-shaped cells are connected to the neuropil<br />
(asterisk) by individual neurites (empty arrowheads).<br />
B). Staining with the pan-neural marker<br />
anti-acetylated tubulin did not reveal<br />
any additional neural structures.<br />
DISCUSSION<br />
The role of the Not gene in metazoan<br />
neurogenesis<br />
The designation of the gene “Not”<br />
refers to the site where its expression<br />
was detected for the first time, namely<br />
in the notochord of the African Clawed<br />
Frog, Xenopus laevis (Gont et al.<br />
1993, von Dassow et al. 1993). The<br />
notochord is a cartilaginous, rod<br />
shaped structure, apomorphic to the<br />
Chordata. It is at least present in some<br />
developmental stages of all chordates,<br />
including the ascidian tadpole larva,<br />
and functions as axial skeleton,<br />
induces the development of the<br />
neural tube, and is thus a key player<br />
in chordate neurogenesis (Stemple<br />
2005). Moreover, Not is expressed in<br />
the neural tube of the mouse, chick,<br />
frog, and zebrafish (Stein and Kessel<br />
1995, Talbot et al. 1995, Yasuo and<br />
Lemaire 2001, Beckers et al. 2007).<br />
Where it has been analyzed in detail,<br />
Not seems to act primarily as a<br />
transcriptional repressor (Yasuo and<br />
Lemaire 2001). In zebrafish, loss-offunction<br />
mutants of floating head (flh;<br />
the zebrafish Not homolog) lack the<br />
notochord altogether, and the somites<br />
fuse below the neural tube (Talbot et al.<br />
1995). Expression studies suggest that<br />
cells lacking flh expression differentiate<br />
into muscle rather than notochordal<br />
tissue (Halpern et al. 1995). Noto,<br />
the Not homolog in the mouse, and<br />
flh repress paraxial mesoderm fate<br />
while maintaining axial mesoderm fate<br />
(Amacher and Kimmel 1998).<br />
Only little is known about the<br />
expression patterns and functions of<br />
Not in non-chordate metazoans. In<br />
Trichoplax adhaerens, the Not gene is<br />
expressed at the bottom of body folds<br />
of intact animals as well as during<br />
wound healing (Martinelli and Spring
74 Submitted manuscript<br />
Chapter IV<br />
2004). In addition, Not expressing cells<br />
in this species, which lacks a nervous<br />
system and even neurons, overlaps<br />
with the site of expression of the<br />
neurotransmitter RFamide (Schuchert<br />
1993). In Drosophila, the Not homolog<br />
90Bre is present in the nervous<br />
system, where it is expressed in the<br />
ventral nerve cord and the posterior<br />
brain anlage of germ band retracted<br />
embryos, as well as in the lateral/<br />
posterior region of the eye/antennal<br />
disc (Dessain and McGinnis 1993).<br />
In the ascidians Halocynthia roretzi<br />
and Ciona intestinalis, Hr-Not and Ci-<br />
Not, respectively, are expressed in the<br />
posterior end of the tail, as well as in<br />
the notochord and a small part of the<br />
anterior neural tube in the larval tailbud<br />
stage (Utsumi et al. 2004). These data<br />
hint towards an ancestral role of Not in<br />
metazoan neurogenesis.<br />
The ring-like expression of TtrNot in<br />
the ciliated region of the apical lobe<br />
in Terebratalia larvae is intriguing.<br />
Noto, the Not ortholog in the mouse,<br />
is known to function in ciliogenesis,<br />
and TtrNot might thus serve a similar<br />
role in our study species. In this<br />
context,it should also be considered<br />
that the putative spiralian homolog<br />
of this larval swimming device, the<br />
prototroch, is underlain, and probably<br />
innervated, by a ring nerve (Wanninger<br />
2009). Accordingly, it is tempting<br />
to speculate that Not may also be<br />
expressed in the prototroch ring nerve<br />
of spiralian trochophore larvae and/<br />
or the prototroch itself. In Terebratalia<br />
transversa, however, we did not find<br />
any corresponding neural structure in<br />
the region of Not expression (Fig. 6).<br />
The larval nervous system of T.<br />
transversa differs in several details<br />
from that of the craniiform brachiopod<br />
Novocrania anomola. In N. anomala,<br />
the apical organ comprises four,<br />
centrally positioned serotonergic flaskshaped<br />
cells that are connected to two<br />
ventral neurites which elongate laterally<br />
along the larval body (Altenburger<br />
and Wanninger 2010). By contrast,<br />
the apical organ of T. transversa has<br />
two sets of flask-shaped cells. Each<br />
set contains four cells and each cell is<br />
connected to the larval anterior neuropil<br />
by a single serotonergic neurite. Due to<br />
these differences, the morphology of<br />
the ancestral brachiopod larval apical<br />
organ remains elusive. However, the<br />
data currently available indicate that an<br />
apical organ comprising serotonergic<br />
flask-shaped cells was part of the<br />
brachiopod ground pattern and most<br />
likely constitutes a morphological<br />
apomorphy of Lophotrochzoa, since<br />
such cells are also found in larval<br />
Entoprocta, Mollusca, Nemertea,<br />
Annelida, and Ectoprocta (Pires and<br />
Woollacott 1997, Shimizu et al. 2000,<br />
Friedrich et al. 2002, Voronezhskaya<br />
et al. 2002, 2003, McDougall et al.<br />
2006, Wanninger et al. 2007, Fuchs<br />
and Wanninger 2008, Chernyshev<br />
and Magarlamov 2010, Nielsen and<br />
Worsaae 2010).<br />
Not expression during gastrulation<br />
and germ layer formation<br />
In all species studied so far, Not<br />
expression starts prior to or at the onset<br />
of gastrulation. This is also the case<br />
in the brachiopod investigated herein,<br />
Terebratalia transversa. In the sea<br />
urchin Strongylocentrotus purpuratus,<br />
Not is expressed in the vegetal plate at<br />
the mesenchyme-blastula stage and<br />
in the secondary mesenchyme, with
Chapter IV<br />
Submitted manuscript<br />
75<br />
expression ceasing after gastrulation<br />
(Peterson et al. 1999). In ascidians,<br />
Not expression starts at the eight<br />
cell stage in all blastomeres and is<br />
thereafter expressed in the posterior<br />
part of the larval tail, the notochord,<br />
and a small part of the anterior neural<br />
tube at the tailbud stage (Utsumi<br />
et al. 2004). Interestingly, we found<br />
TtrNot being solely expressed in the<br />
ectoderm of T. transversa, while their<br />
homologs are expressed in all three<br />
germ layers during Xenopus and<br />
ascidian embryogenesis (von Dassow<br />
et al. 1993, Utsumi et al. 2004).<br />
Apart from the development of the<br />
nervous system, the notochord,<br />
and various germ layers, Not is also<br />
responsible for left/right patterning<br />
in the mouse, where it is expressed<br />
in the “node”, i.e., the organizer of<br />
gastrulation (Beckers et al. 2007).<br />
In the sea urchins Hemicentrotus<br />
pulcherimus and Strongylocentrotus<br />
purpuratus, Not is expressed in the<br />
archenteron of the gastrula and in<br />
the mesoderm of the right coelomic<br />
pouch of two-armed pluteus larvae,<br />
were it is likewise involved in left/right<br />
determination (Peterson et al. 1999,<br />
Hibino et al. 2006).<br />
The current data suggest an overall role<br />
of Not in gastrulation as well as germ<br />
layer and nervous system patterning.<br />
Whether Not was used in specification<br />
of all three germ layers in Urbilateria<br />
(as exemplified in the ascidians and<br />
Xenopus) or whether its ancestral role<br />
was in ectoderm patterning alone (as<br />
in Terebratalia) remains to be revealed<br />
by future comparative studies. In<br />
any case, it appears that the Not<br />
gene has been co-opted into several<br />
other functions during evolution of<br />
respective metazoan (deuterostome)<br />
lineages, such as notochord formation<br />
in chordates and left/right patterning<br />
in ambulacrarians (sea urchin) and<br />
vertebrates (mouse).<br />
The role of Cdx in metazoan<br />
development<br />
Cdx is a member of the ParaHox cluster<br />
in which three genes are linked in a<br />
manner reminiscent of the Hox genes,<br />
with the gene order 3’-Gsx-Xlox-Cdx-5’<br />
(Brooke et al. 1998). Compared to Hox<br />
genes, ParaHox genes seem to be<br />
much more evolutionary labile, since<br />
they do not appear together in all<br />
species investigated and sometimes<br />
they are not clustered (Ferrier and<br />
Holland 2002).<br />
Cdx expression patterns are known<br />
from several animal phyla and there is<br />
a wide range of tissues in which Cdx is<br />
expressed (Fröbius and Seaver 2006).<br />
A gene related to Cdx is present in<br />
the proposed bilaterian sister group,<br />
the cnidarian Nematostella vectensis<br />
(Chourrout et al. 2006, Ryan et al. 2006,<br />
2007). Cdx was first characterized as a<br />
posterior patterning gene in Drosophila<br />
melanogaster (Mlodzik et al. 1985)<br />
and it appears to serve a similar role<br />
in a number of other taxa including<br />
various arthropods, the nematode<br />
Caenorhabditis elegans, and the basal<br />
gastropod mollusk Patella vulgata<br />
(Waring and Kenyon 1991, Xu et al.<br />
1994, Schulz et al. 1998, Abzhanov<br />
and Kaufman 2000, Dearden and<br />
Akam 2001, Rabet et al. 2001, Copf et<br />
al. 2003, Le Gouar et al. 2003, 2004,<br />
Shinmyo et al. 2005, Olesnicky et al.<br />
2006). In the annelids Platynereis<br />
dumerilii, Nereis virens, Tubifex<br />
tubifex, and Capitella sp., Cdx has an
76 Submitted manuscript<br />
Chapter IV<br />
anterior and a posterior expression<br />
domain (Fröbius and Seaver 2006,<br />
Matsuo and Shimizu 2006, Kulakova<br />
et al. 2008, Hui et al. 2009).<br />
The expression pattern of Cdx in<br />
Terebratalia transversa shows some<br />
similarity to that of Platynereis dumerilii.<br />
In both species Cdx is expressed in the<br />
ectoderm at the onset of gastrulation. In<br />
P. dumerilii, the ectodermal expression<br />
of PduCdx encircles the posterior<br />
portion of the slit-like blastopore and<br />
extends from there anteriorly along its<br />
edges (de Rosa et al. 2005). PduCdx<br />
continues to be expressed in the<br />
posterior part of the trochophore larva<br />
in the posterior midgut and hindgut<br />
(Hui et al. 2009). Expression of Cdx in<br />
the gut is also found in the sea urchin<br />
Strongylocentrotus purpuratus, the<br />
lancelet Brachiostoma floridae, and<br />
the mouse Mus musculus (Duprey<br />
et al. 1988, Brooke et al. 1998,<br />
Arnone et al. 2006). We did not find<br />
expression of TtrCdx in the larval gut<br />
of Terebratalia transversa, which might<br />
be due to the fact that those larvae are<br />
lecithotrophic and that metamorphosis<br />
is catastrophic, i.e., that all major larval<br />
tissues degenerate after settlement<br />
(Stricker and Reed 1985a, 1985b).<br />
Since we did not investigate feeding<br />
juveniles with a functional gut, the role<br />
of TtrCdx in gut formation remains<br />
elusive.<br />
Concerning the role of Cdx in the<br />
protostome-deuterostome ancestor<br />
(PDA), two major hypotheses are<br />
currently discussed. Either, expression<br />
in the PDA might have been in an<br />
anterior and a posterior domain of the<br />
nervous system, as in recent acoels<br />
as well as the lophotrochozoans<br />
Platynereis dumerilii, Capitella sp.,<br />
Tubifex tubifex, and Patella vulgata<br />
(Le Gouar et al. 2003, de Rosa et al.<br />
2005, Matsuo et al. 2005, Fröbius and<br />
Seaver 2006, Hejnol and Martindale<br />
2008). Expression in the hindgut and<br />
posterior tissues of recent animals<br />
would thus have been co-opted. Or,<br />
Cdx expression in the PDA was in<br />
posterior tissues and a dissociation<br />
of Cdx from the ParaHox cluster in<br />
Lophotrochozoa allowed for its cooption<br />
into the anterior domain of the<br />
nervous system, as is the case in<br />
acoels and some lophotrochozoans<br />
(Hui et al. 2009). In this respect it<br />
would be interesting to focus future<br />
investigations on the genomic<br />
arrangement and the expression of<br />
the respective Gsx and Xlox genes of<br />
the ParaHox cluster in brachiopods.<br />
ACKNOWLEDGEMENTS<br />
We thank the Friday Harbor<br />
Laboratories and especially Billie<br />
Swalla (University of Washington) for<br />
providing lab space and assistance in<br />
rearing Terebratalia transversa. Olga<br />
Lévai (Leica Microsystems, Mannheim,<br />
Germany) is thanked for providing the<br />
SP5 confocal system that was used for<br />
the scans upon which Fig. 6 is based.<br />
We are grateful to Marta Chiodin<br />
(University of Barcelona) for guidance<br />
in lab procedures. Bernard M. Degnan<br />
(University of Queensland) is thanked<br />
for sharing previously unpublished<br />
sequence data of the demosponge<br />
Amphimedon queenslandica. This<br />
study was funded by the Danish<br />
Agency for Science, Technology and<br />
Innovation (grant no. 645-06-0294<br />
to AW). Research in the lab of AW<br />
and PM was further supported by<br />
the EU-funded Marie Curie Network
Chapter IV<br />
Submitted manuscript<br />
77<br />
MOLMORPH (contract grant number<br />
MEST-CT-2005-020542). PM is<br />
grateful to the Spanish Ministerio<br />
de Ciencia e Innovación and the<br />
Generalitat de Catalunya for financial<br />
support.<br />
REFERENCES<br />
Abdelkhalek, H. B., A. Beckers,<br />
K. Schuster-Gossler, M.<br />
N. Pavlova, H. Burkhardt,<br />
H. Lickert, J. Rossant, R.<br />
Reinhardt, L. C. Schalkwyk,<br />
I. Müller, B. G. Herrmann,<br />
M. Ceolin, R. Rivera-Pomar,<br />
and A. Gossler. 2004. The<br />
mouse homeobox gene Not is<br />
required for caudal notochord<br />
development and affected by<br />
the truncate mutation. Genes<br />
Dev 18:1725-1736.<br />
Abzhanov, A., and T. C. Kaufman.<br />
2000. Embryonic expression<br />
patterns of the Hox genes of the<br />
crayfish Procambarus clarkii<br />
(Crustacea, Decapoda). Evol<br />
Dev 2:271-283.<br />
Altenburger, A., and A. Wanninger.<br />
2010. Neuromuscular<br />
development in Novocrania<br />
anomala: evidence for the<br />
presence of serotonin and<br />
a spiralian-like apical organ<br />
in lecithotrophic brachiopod<br />
larvae. Evol Dev 12:16-24.<br />
Amacher, S. L., and C. B. Kimmel.<br />
1998. Promoting notochord<br />
fate and repressing muscle<br />
development in zebrafish axial<br />
mesoderm. Development<br />
125:1397-1406.<br />
Arnone, M. I., F. Rizzo, R. Annunciata,<br />
R. A. Cameron, K. J. Peterson,<br />
and P. Martínez. 2006. Genetic<br />
organization and embryonic<br />
expression of the ParaHox<br />
genes in the sea urchin S.<br />
purpuratus: Insights into the<br />
relationship between clustering<br />
and colinearity. Dev Biol 300:63-<br />
73.<br />
Beckers, A., L. Alten, C. Viebahn,<br />
P. Andre, and A. Gossler.<br />
2007. The mouse homeobox<br />
gene Noto regulates node<br />
morphogenesis, notochordal<br />
ciliogenesis, and left-right<br />
patterning. Proc Natl Acad Sci<br />
USA 104:15765-15770.<br />
Brooke, N. M., J. Garcia-Fernandez,<br />
and P. W. H. Holland. 1998.<br />
The ParaHox gene cluster is an<br />
evolutionary sister of the Hox<br />
gene cluster. Nature 392:920-<br />
922.<br />
Chernyshev, A. V., and T. Y.<br />
Magarlamov. 2010. The first<br />
data on the nervous system<br />
of hoplonemertean larvae<br />
(Nemertea, Hoplonemertea).<br />
Gen Biol 430:48-50.<br />
Chourrout, D., F. Delsuc, P. Chourrout,<br />
R. B. Edvardsen, F. Rentzsch,<br />
E. Renfer, M. F. Jensen, B.<br />
Zhu, P. de Jong, R. E. Steele,<br />
and U. Technau. 2006. Minimal<br />
ProtoHox cluster inferred from<br />
bilaterian and cnidarian Hox<br />
complements. Nature 442:684-<br />
687.<br />
Cohen, B. L., and A. Weydmann.<br />
2005. Molecular evidence that<br />
phoronids are a subtaxon of<br />
brachiopods (Brachiopoda:<br />
Phoronata) and that genetic<br />
divergence of metazoan phyla<br />
began long before the early
78 Submitted manuscript<br />
Chapter IV<br />
Cambrian. Org Divers Evol<br />
5:253-273.<br />
Copf, T., N. Rabet, S. E. Celniker,<br />
and M. Averof. 2003. Posterior<br />
patterning genes and the<br />
identification of a unique<br />
body region in the brine<br />
shrimp Artemia franciscana.<br />
Development 130:5915-5927.<br />
Copf, T., R. Schröder, and M. Averof.<br />
2004. Ancestral role of caudal<br />
genes in axis elongation and<br />
segmentation. Proc Natl Acad<br />
Sci USA 101:17711-17715.<br />
de Rosa, R., J. K. Grenier, T. Andreeva,<br />
C. E. Cook, A. Adoutte, M.<br />
Akam, S. B. Carroll, and G.<br />
Balavoine. 1999. Hox genes in<br />
brachiopods and priapulids and<br />
protostome evolution. Nature<br />
399:772-776.<br />
de Rosa, R., B. Prud’homme, and G.<br />
Balavoine. 2005. caudal and<br />
even-skipped in the annelid<br />
Platynereis dumerilii and the<br />
ancestry of posterior growth.<br />
Evol Dev 7:574-587.<br />
Dearden, P. K., and M. Akam. 2001.<br />
Early embryo patterning in the<br />
grasshopper, Schistocerca<br />
gregaria:<br />
wingless,<br />
decapentaplegic and caudal<br />
expression. Development<br />
128:3435-3444.<br />
Dessain, S., and W. McGinnis. 1993.<br />
Drosophila homeobox genes.<br />
Adv Dev Biochem 2:1-55.<br />
Duprey, P., K. Chowdhury, G. R.<br />
Dressler, R. Balling, D. Simon, J.<br />
L. Guenet, and P. Gruss. 1988.<br />
A mouse gene homologous to<br />
the Drosophila gene caudal is<br />
expressed in epithelial cells<br />
from the embryonic intestine.<br />
Genes Dev 2:1647-1654.<br />
Epstein, M., G. Pillemer, R. Yelin, J. K.<br />
Yisraeli, and A. Fainsod. 1997.<br />
Patterning of the embryo along<br />
the anterior-posterior axis:<br />
the role of the caudal genes.<br />
Development 124:3805-3814.<br />
Ferrier, D. E. K., and P. W. H. Holland.<br />
2002. Ciona intestinalis<br />
ParaHox genes: evolution of<br />
Hox/ParaHox cluster integrity,<br />
developmental mode, and<br />
temporal colinearity. Mol<br />
Phylogenet Evol 24:412-417.<br />
Friedrich, S., A. Wanninger, M.<br />
Bruckner, and G. Haszprunar.<br />
2002. Neurogenesis in the<br />
mossy chiton, Mopalia muscosa<br />
(Gould) (Polyplacophora):<br />
Evidence against molluscan<br />
metamerism. J Morphol<br />
253:109-117.<br />
Fröbius, A., and E. Seaver. 2006.<br />
ParaHox gene expression in the<br />
polychaete annelid Capitella sp.<br />
I. Dev Genes Evol 216:81-88.<br />
Fuchs, J., and A. Wanninger.<br />
2008. Reconstruction of<br />
the neuromuscular system<br />
of the swimming-type larva<br />
of Loxosomella atkinsae<br />
(Entoprocta) as inferred by<br />
fluorescence labelling and<br />
confocal microscopy. Org<br />
Divers Evol 8:325-335.<br />
Gauchat, D., F. Mazet, C. Berney,<br />
M. Schummer, S. Kreger, J.<br />
Pawlowski, and B. Galliot. 2000.<br />
Evolution of Antp-class genes<br />
and differential expression of<br />
Hydra Hox/ParaHox genes in<br />
anterior patterning. Proc Natl<br />
Acad Sci USA 97:4493-4498.<br />
Gont, L., H. Steinbeisser, B. Blumberg,
Chapter IV<br />
Submitted manuscript<br />
79<br />
and E. de Robertis. 1993. Tail<br />
formation as a continuation of<br />
gastrulation: the multiple cell<br />
populations of the Xenopus<br />
tailbud derive from the late<br />
blastopore lip. Development<br />
119:991-1004.<br />
Gont, L. K., A. Fainsod, S.-H. Kim,<br />
and E. M. de Robertis.<br />
1996. Overexpression of the<br />
homeobox gene Xnot-2 leads<br />
to notochord formation in<br />
Xenopus. Dev Biol 174:174-<br />
178.<br />
Halpern, M. E., C. Thisse, R. K. Ho,<br />
B. Thisse, B. Riggleman, B.<br />
Trevarrow, E. S. Weinberg,<br />
J. H. Postlethwait, and C. B.<br />
Kimmel. 1995. Cell-autonomous<br />
shift from axial to paraxial<br />
mesodermal development in<br />
zebrafish floating head mutants.<br />
Development 121:4257-4264.<br />
Hejnol, A., and M. Q. Martindale. 2008.<br />
Acoel development indicates<br />
the independent evolution of<br />
the bilaterian mouth and anus.<br />
Nature 456:382-386.<br />
Hejnol, A., M. Obst, A. Stamatakis,<br />
M. Ott, G. W. Rouse, G. D.<br />
Edgecombe, P. Martinez, J.<br />
Baguñà, X. Bailly, U. Jondelius,<br />
M. Wiens, W. E. G. Müller, E.<br />
Seaver, W. C. Wheeler, M. Q.<br />
Martindale, G. Giribet, and<br />
C. W. Dunn. 2009. Assessing<br />
the root of bilaterian animals<br />
with scalable phylogenomic<br />
methods. Proc R Soc B<br />
276:4261-4270.<br />
Hibino, T., Y. Ishii, M. Levin, and<br />
A. Nishino. 2006. Ion flow<br />
regulates left–right asymmetry<br />
in sea urchin development. Dev<br />
Genes Evol 216:265-276.<br />
Hoskins, R. A., J. W. Carlson, C.<br />
Kennedy, D. Acevedo, M.<br />
Evans-Holm, E. Frise, K. H.<br />
Wan, S. Park, M. Mendez-<br />
Lago, F. Rossi, A. Villasante,<br />
P. Dimitri, G. H. Karpen, and S.<br />
E. Celniker. 2007. Sequence<br />
finishing and mapping of<br />
Drosophila melanogaster<br />
heterochromatin. Science<br />
316:1625-1628.<br />
Hueber, S. D., and I. Lohmann. 2008.<br />
Shaping segments: Hox gene<br />
function in the genomic age.<br />
Bioessays 30:965-979.<br />
Hui, J., F. Raible, N. Korchagina, N.<br />
Dray, S. Samain, G. Magdelenat,<br />
C. Jubin, B. Segurens, G.<br />
Balavoine, D. Arendt, and D.<br />
Ferrier. 2009. Features of the<br />
ancestral bilaterian inferred from<br />
Platynereis dumerilii ParaHox<br />
genes. BMC Biol 7:43.<br />
Knezevic, V., M. Ranson, and S.<br />
Mackem. 1995. The organizerassociated<br />
chick homeobox<br />
gene, Gnot1, is expressed<br />
before gastrulation and<br />
regulated synergistically by<br />
activin and retinoic acid. Dev<br />
Biol 171:458-470.<br />
Kulakova, M., C. Cook, and T.<br />
Andreeva. 2008. ParaHox<br />
gene expression in larval and<br />
postlarval development of<br />
the polychaete Nereis virens<br />
(Annelida, Lophotrochozoa).<br />
BMC Dev Biol 8:61.<br />
Lanfear, R., and L. Bromham. 2008.<br />
Statistical tests between<br />
competing hypotheses of Hox<br />
cluster evolution. Syst. Biol.<br />
57:708-718.
80 Submitted manuscript<br />
Chapter IV<br />
Larroux, C., G. N. Luke, P. Koopman,<br />
D. S. Rokhsar, S. M. Shimeld,<br />
and B. M. Degnan. 2008.<br />
Genesis and expansion of<br />
metazoan transcription factor<br />
gene classes. Mol Biol Evol<br />
25:980-996.<br />
Le Gouar, M., N. Lartillot, A. Adoutte,<br />
and M. Vervoort. 2003.<br />
The expression of a caudal<br />
homologue in a mollusc, Patella<br />
vulgata. Gene Expr Patterns.<br />
3:35-37.<br />
Macdonald, P. M., and G. Struhl. 1986.<br />
A molecular gradient in early<br />
Drosophila embryos and its role<br />
in specifying the body pattern.<br />
Nature 324:537-545.<br />
Martinelli, C., and J. Spring. 2004.<br />
Expression pattern of the<br />
homeobox gene Not in the<br />
basal metazoan Trichoplax<br />
adhaerens. Gene Expr Patterns.<br />
4:443-447.<br />
Matsuo, K., and T. Shimizu. 2006.<br />
Embryonic expression of a<br />
decapentaplegic gene in the<br />
oligochaete annelid Tubifex<br />
tubifex. Gene Expr Patterns.<br />
6:800-806.<br />
Matsuo, K., H. Yoshida, and T. Shimizu.<br />
2005. Differential expression<br />
of caudal and dorsal genes in<br />
the teloblast lineages of the<br />
oligochaete annelid Tubifex<br />
tubifex. Dev Genes Evol<br />
215:238-247.<br />
McDougall, C., W.-C. Chen, S.<br />
Shimeld, and D. Ferrier.<br />
2006. The development of<br />
the larval nervous system,<br />
musculature and ciliary bands<br />
of Pomatoceros lamarckii<br />
(Annelida): heterochrony in<br />
polychaetes. Front Zool 3:16.<br />
McGinnis, W., M. S. Levine, E. Hafen,<br />
A. Kuroiwa, and W. J. Gehring.<br />
1984. A conserved DNA<br />
sequence in homoeotic genes<br />
of the Drosophila Antennapedia<br />
and bithorax complexes. Nature<br />
308:428-433.<br />
Mlodzik, M., A. Fjose, and W. J.<br />
Gehring. 1985. Isolation of<br />
caudal, a Drosophila homeo<br />
box-containing gene with<br />
maternal expression, whose<br />
transcripts form a concentration<br />
gradient at the pre-blastoderm<br />
stage. EMBO J 4:2961-2969.<br />
Nielsen, C. 2002. Ciliary filter-feeding<br />
structures in adult and larval<br />
gymnolaemate bryozoans.<br />
Invertebr Biol 121:255-261.<br />
Nielsen, C., and K. Worsaae. 2010.<br />
Structure and occurrence of<br />
cyphonautes larvae (Bryozoa,<br />
Ectoprocta). J Morphol<br />
271:1094-1109.<br />
Odenthal, J., P. Haffter, E. Vogelsang,<br />
M. Brand, F. van Eeden, M.<br />
Furutani-Seiki, M. Granato,<br />
M. Hammerschmidt, C.<br />
Heisenberg, Y. Jiang, D. Kane,<br />
R. Kelsh, M. Mullins, R. Warga,<br />
M. Allende, E. Weinberg,<br />
and C. Nüsslein-Volhard.<br />
1996. Mutations affecting the<br />
formation of the notochord<br />
in the zebrafish, Danio rerio.<br />
Development 123:103-115.<br />
Olesnicky, E. C., A. E. Brent, L. Tonnes,<br />
M. Walker, M. A. Pultz, D.<br />
Leaf, and C. Desplan. 2006. A<br />
caudal mRNA gradient controls<br />
posterior development in the<br />
wasp Nasonia. Development<br />
133:3973-3982.
Chapter IV<br />
Submitted manuscript<br />
81<br />
Paps, J., J. Baguñà, and M. Riutort.<br />
2009. Lophotrochozoa internal<br />
phylogeny: new insights from an<br />
up-to-date analysis of nuclear<br />
ribosomal genes. Proc R Soc B<br />
276:1245-1254.<br />
Peterson, K. J., Y. Harada, R. A.<br />
Cameron, and E. H. Davidson.<br />
1999. Expression pattern of<br />
Brachyury and Not in the sea<br />
urchin: comparative implications<br />
for the origins of mesoderm in<br />
the basal deuterostomes. Dev<br />
Biol 207:419-431.<br />
Pires, A., and R. M. Woollacott. 1997.<br />
Serotonin and dopamine have<br />
opposite effects on phototaxis<br />
in larvae of the bryozoan Bugula<br />
neritina. Biol Bull 192:399-409.<br />
Quiquand, M., N. Yanze, J. Schmich,<br />
V. Schmid, B. Galliot, and S.<br />
Piraino. 2009. More constraint<br />
on ParaHox than Hox gene<br />
families in early metazoan<br />
evolution. Dev Biol 328:173-<br />
187.<br />
Rabet, N., J.-M. Gibert, É. QuÉinnec,<br />
J. S. Deutsch, and E. Mouchel-<br />
Vielh. 2001. The caudal gene of<br />
the barnacle Sacculina carcini<br />
is not expressed in its vestigial<br />
abdomen. Dev Genes Evol<br />
211:172-178.<br />
Ryan, J., P. Burton, M. Mazza, G.<br />
Kwong, J. Mullikin, and J.<br />
Finnerty. 2006. The cnidarianbilaterian<br />
ancestor possessed<br />
at least 56 homeoboxes:<br />
evidence from the starlet<br />
sea anemone, Nematostella<br />
vectensis. Genome Biol 7:R64.<br />
Ryan, J. F., M. E. Mazza, K. Pang,<br />
D. Q. Matus, A. D. Baxevanis,<br />
M. Q. Martindale, and J. R.<br />
Finnerty. 2007. Pre-bilaterian<br />
origins of the Hox cluster and<br />
the Hox code: evidence from<br />
the sea anemone, Nematostella<br />
vectensis. PLoS ONE 2.<br />
Schuchert, P. 1993. Trichoplax<br />
adhaerens (phylum Placozoa)<br />
has cells that react with<br />
antibodies against the<br />
neuropeptide RFamide. Acta<br />
Zool 74.<br />
Schulz, C., R. Schröder, B. Hausdorf,<br />
C. Wolff, and D. Tautz. 1998. A<br />
caudal homologue in the short<br />
germ band beetle Tribolium<br />
shows similarities to both, the<br />
Drosophila and the vertebrate<br />
caudal expression patterns.<br />
Dev Genes Evol 208:283-289.<br />
Scott, M. P., and A. J. Weiner. 1984.<br />
Structural relationships among<br />
genes that control development:<br />
sequence homology between<br />
the antennapedia, ultrabithorax,<br />
and fushi tarazu loci of<br />
Drosophila. Proc Natl Acad Sci<br />
USA 81:4115-4119.<br />
Shimizu, K., E. Hunter, and N.<br />
Fusetani. 2000. Localisation<br />
of biogenic amines in larvae<br />
of Bugula neritina (Bryozoa:<br />
Cheilostomatida) and their<br />
effects on settlement. Mar Biol<br />
136:1-9.<br />
Shinmyo, Y., T. Mito, T. Matsushita,<br />
I. Sarashina, K. Miyawaki, H.<br />
Ohuchi, and S. Noji. 2005.<br />
caudal is required for gnathal<br />
and thoracic patterning and<br />
for posterior elongation in the<br />
intermediate-germband cricket<br />
Gryllus bimaculatus. Mech Dev<br />
122:231-239.<br />
Srivastava, M., O. Simakov, J.
82 Submitted manuscript<br />
Chapter IV<br />
Chapman, B. Fahey, M. E.<br />
A. Gauthier, T. Mitros, G.<br />
S. Richards, C. Conaco, M.<br />
Dacre, U. Hellsten, C. Larroux,<br />
N. H. Putnam, M. Stanke, M.<br />
Adamska, A. Darling, S. M.<br />
Degnan, T. H. Oakley, D. C.<br />
Plachetzki, Y. Zhai, M. Adamski,<br />
A. Calcino, S. F. Cummins, D.<br />
M. Goodstein, C. Harris, D. J.<br />
Jackson, S. P. Leys, S. Shu,<br />
B. J. Woodcroft, M. Vervoort,<br />
K. S. Kosik, G. Manning, B. M.<br />
Degnan, and D. S. Rokhsar.<br />
2010. The Amphimedon<br />
queenslandica genome and the<br />
evolution of animal complexity.<br />
Nature 466:720-726.<br />
Stein, S., and M. Kessel. 1995. A<br />
homeobox gene involved in<br />
node, notochord and neural<br />
plate formation of chick<br />
embryos. Mech Dev 49:37-48.<br />
Stein, S., K. Niß, and M. Kessel. 1996.<br />
Differential activation of the<br />
clustered homeobox genes<br />
CNOT2 and CNOT1 during<br />
notogenesis in the chick. Dev<br />
Biol 180:519-533.<br />
Stemple, D. L. 2005. Structure and<br />
function of the notochord: an<br />
essential organ for chordate<br />
development. Development<br />
132:2503-2512.<br />
Stricker, S. A., and C. G. Reed. 1985a.<br />
The ontogeny of shell secretion<br />
in Terebratalia transversa<br />
(Brachiopoda, Articulata). 1.<br />
Development of the mantle. J<br />
Morphol 183:233-250.<br />
Stricker, S. A., and C. G. Reed. 1985b.<br />
The ontogeny of shell secretion<br />
in Terebratalia transversa<br />
(Brachiopoda, Articulata). 2.<br />
formation of the protegulum<br />
and juvenile shell. J Morphol<br />
183:251-271.<br />
Talbot, W. S., B. Trevarrow, M. E.<br />
Halpern, A. E. Melby, G.<br />
Farr, J. H. Postlethwait, T.<br />
Jowett, C. B. Kimmel, and D.<br />
Kimelman. 1995. A homeobox<br />
gene essential for zebrafish<br />
notochord development. Nature<br />
378:150-157.<br />
Utsumi, N., Y. Shimojima, and H. Saiga.<br />
2004. Analysis of ascidian<br />
Not genes highlights their<br />
evolutionarily conserved and<br />
derived features of structure<br />
and expression in development.<br />
Dev Genes Evol 214:460-465.<br />
von Dassow, G., J. E. Schmidt, and<br />
D. Kimelman. 1993. Induction<br />
of the Xenopus organizer:<br />
expression and regulation of<br />
Xnot, a novel FGF and activinregulated<br />
homeo box gene.<br />
Genes Dev 7:355-366.<br />
Voronezhskaya, E. E., E. B. Tsitrin,<br />
and L. P. Nezlin. 2003.<br />
Neuronal development in<br />
larval polychaete Phyllodoce<br />
maculata (Phyllodocidae). J<br />
Comp Neurol. 455:299-309.<br />
Voronezhskaya, E. E., S. A. Tyurin,<br />
and L. P. Nezlin. 2002. Neuronal<br />
development in larval chiton<br />
Ischnochiton hakodadensis<br />
(Mollusca: Polyplacophora).<br />
J Submicrosc Cytol Pathol<br />
444:25-38.<br />
Wanninger, A. 2009. Shaping the<br />
things to come: ontogeny<br />
of<br />
lophotrochozoan<br />
neuromuscular systems and<br />
the Tetraneuralia concept. Biol.<br />
Bull. 216:293-306.
Chapter IV<br />
Submitted manuscript<br />
83<br />
Wanninger, A., J. Fuchs, and G.<br />
Haszprunar. 2007. Anatomy<br />
of the serotonergic nervous<br />
system of an entoproct creepingtype<br />
larva and its phylogenetic<br />
implications. Invertebr Biol<br />
126:268-278.<br />
Waring, D. A., and C. Kenyon.<br />
1991. Regulation of cellular<br />
responsiveness to inductive<br />
signals in the developing C.<br />
elegans nervous system.<br />
Nature 350:712-715.<br />
Williams, A., and S. J. Carlson. 2007.<br />
Affinities of brachiopods and<br />
trends in their evolution in P.<br />
A. Selden, ed. Treatise on<br />
Invertebrate Paleontology,<br />
Part H, Brachiopoda, Revised.<br />
Geological Society of America<br />
& Paleontological Institute,<br />
Boulder, Colorado & Lawrence,<br />
Kansas.<br />
Xu, X., P. X. Xu, and Y. Suzuki.<br />
1994. A maternal homeobox<br />
gene, Bombyx caudal, forms<br />
both mRNA and protein<br />
concentration gradients<br />
spanning anteroposterior<br />
axis during gastrulation.<br />
Development 120:277-285.<br />
Yasuo, H., and P. Lemaire. 2001.<br />
Role of goosecoid, Xnot<br />
and Wnt antagonists in the<br />
maintenance of the notochord<br />
genetic programme in Xenopus<br />
gastrulae. Development<br />
128:3783-3793.