NCCLS Guidelines
NCCLS Guidelines
NCCLS Guidelines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
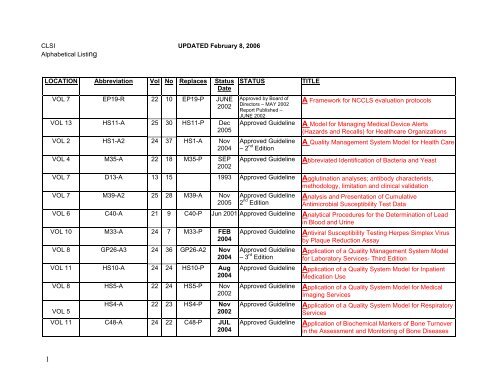
CLSI UPDATED February 8, 2006<br />
Alphabetical Listing<br />
LOCATION Abbreviation Vol No Replaces Status<br />
Date<br />
VOL 7 EP19-R 22 10 EP19-P JUNE<br />
2002<br />
VOL 13 HS11-A 25 30 HS11-P Dec<br />
2005<br />
VOL 2 HS1-A2 24 37 HS1-A Nov<br />
2004<br />
VOL 4 M35-A 22 18 M35-P SEP<br />
2002<br />
STATUS<br />
Approved by Board of<br />
Directors – MAY 2002<br />
Report Published –<br />
JUNE 2002<br />
Approved Guideline<br />
Approved Guideline<br />
– 2 nd Edition<br />
Approved Guideline<br />
TITLE<br />
A Framework for <strong>NCCLS</strong> evaluation protocols<br />
A Model for Managing Medical Device Alerts<br />
(Hazards and Recalls) for Healthcare Organizations<br />
A Quality Management System Model for Health Care<br />
Abbreviated Identification of Bacteria and Yeast<br />
VOL 7 D13-A 13 15 1993 Approved Guideline Agglutination analyses; antibody characterists,<br />
methodology, limitation and clinical validation<br />
VOL 7 M39-A2 25 28 M39-A Nov<br />
2005<br />
Approved Guideline<br />
2 nd Edition<br />
Analysis and Presentation of Cumulative<br />
Antimicrobial Susceptibility Test Data<br />
VOL 6 C40-A 21 9 C40-P Jun 2001 Approved Guideline Analytical Procedures for the Determination of Lead<br />
in Blood and Urine<br />
VOL 10 M33-A 24 7 M33-P FEB<br />
2004<br />
VOL 8 GP26-A3 24 36 GP26-A2 Nov<br />
2004<br />
VOL 11 HS10-A 24 24 HS10-P Aug<br />
2004<br />
VOL 8 HS5-A 22 24 HS5-P Nov<br />
2002<br />
VOL 5<br />
HS4-A 22 23 HS4-P Nov<br />
2002<br />
VOL 11 C48-A 24 22 C48-P JUL<br />
2004<br />
Approved Guideline<br />
Approved Guideline<br />
– 3 rd Edition<br />
Approved Guideline<br />
Approved Guideline<br />
Approved Guideline<br />
Approved Guideline<br />
Antiviral Susceptibility Testing Herpes Simplex Virus<br />
by Plaque Reduction Assay<br />
Application of a Quality Management System Model<br />
for Laboratory Services- Third Edition<br />
Application of a Quality System Model for Inpatient<br />
Medication Use<br />
Application of a Quality System Model for Medical<br />
Imaging Services<br />
Application of a Quality System Model for Respiratory<br />
Services<br />
Application of Biochemical Markers of Bone Turnover<br />
in the Assessment and Monitoring of Bone Diseases<br />
1
CLSI Updated February 8, 2006<br />
Alphabetical Listing<br />
LOCATION Abbreviation Vol No Replaces Status<br />
Date<br />
STATUS<br />
TITLE<br />
VOL 10 I/LA23-A 24 16 I/L23-P 5/04 Approved Guideline Assessing the Quality of Immunoassay System:<br />
Radioimmunoassays and Enzyme, Fluorescence, and<br />
Luminescence Immunoassays; Approved Guideline<br />
VOL 8 LA1-A2 14 17 LA1-A Dec<br />
1994<br />
VOL 5 GP-10A 15 19 GP10-T DEC<br />
1995<br />
VOL 7 GP29-A 22 26 GPA29-P Dec<br />
2002<br />
Approved Guideline<br />
Approved Guideline<br />
Approved Guideline<br />
Assessing the Quality of Radioimmunoassay Systems<br />
– Second Edition<br />
Assessment of the Clinical Accuracy of Laboratory<br />
Tests Using Receiver Operating Characteristic<br />
(ROC)Plots<br />
Assessment of Laboratory Tests When Proficiency<br />
Testing is not Available<br />
VOL 3 GP11-A 18 14 GP11-T Nov-98 Approved Guideline Basic cost accounting for clinical services<br />
VOL 9 LA4-A4 23 21 LA3-A4 Jul 2003 Approved Standard –<br />
4 th Edition<br />
VOL 4 C46-A 21 14 C46-P Sep<br />
2001<br />
Approved Guideline<br />
Blood Collection on Filter Paper for Newborn<br />
Screening Programs<br />
Blood gas and pH analysis & related measurements<br />
VOL 13 H56-P 25 20 Aug 2005 Proposed Guideline Body Fluid Analysis for Cellular Composition<br />
VOL 3 H38-P 19 7 Apr-99 Proposed Standard Calibration and quality control of automated<br />
hematology analyzers<br />
VOL 9 H42-A 17 18 42-T Dec 1998 Approved Guideline Clinical Applications of Flow Cytometry: Quality<br />
Assurance and Immunophenotyping of Lymphocytes<br />
VOL 9 H43-A 17 18 43-P June<br />
1998<br />
Approved Guideline<br />
Clinical Applications of Flow Cytometry:<br />
Immunophenotyping of Leukemic Cells<br />
2
CLSI UPDATED February 8, 2006<br />
LOCATION Abbreviation Vol No Replaces Status<br />
Date<br />
VOL 2 I/LA 21-A 22 9 I/LA21-P June<br />
2002<br />
VOL 5 GP17-A2 24 13 GP17-A APRIL<br />
2004<br />
VOL 4 GP2-A4 22 5 GP2-A3 April<br />
2002<br />
VOL 7 GP5-A2 22 3 GP5-A March<br />
2002<br />
VOL 10 M36-A 24 6 M36-P FEB<br />
2004<br />
VOL 7 H21-A4 23 35 H21-A3 DEC<br />
2003<br />
VOL 12 MM13-A 25 31 MM13-P Dec<br />
2005<br />
VOL 4 GP22-A2 24 35 GP22-A Nov<br />
2004<br />
STATUS<br />
Approved<br />
Guideline<br />
Approved Guideline-<br />
2 ND Edition<br />
Approved Guideline<br />
4 th Edition<br />
Approved Guideline<br />
Second Edition<br />
Approved Guideline<br />
Approved Guideline<br />
– 4 th Edition<br />
Approved Guideline<br />
Approved Guideline<br />
– 2 nd Edition<br />
TITLE<br />
Clinical evaluation of immunoassays<br />
Clinical Laboratory Safety<br />
Clinical laboratory technical procedure manuals<br />
Clinical laboratory waste management<br />
Clinical Use and Interpretation of Serologic Tests for<br />
Toxoplasma gondii<br />
Collection, transport, and processing of blood specimens for<br />
coagulation testing plasma – based coagulation assays<br />
Collection, Transport, Preparation, and Storage of<br />
Specimens for Molecular Methods<br />
Continuous quality improvement; Integrating Five Key<br />
Quality System Components<br />
VOL 2 C 39-A 18 23 C39-P Oct-99 Approved<br />
Standard<br />
Designated comparison method for the measurement<br />
of ionized calcium in serum<br />
VOL 5 I/LA22-P 20 10 2000 Proposed Guideline Detection of Anticardiolipin Antibodies by ELISA<br />
VOL 8 H17-A 18 19 H17-P DEC<br />
1998<br />
Approved Standard<br />
Determination of Serum Ion, Total Iron-Binding<br />
Capacity and Percent Transferrin Saturation<br />
3
CLSI UPDATED February 8, 2006<br />
LOCATION Abbreviation Vol No Replaces Status<br />
Date<br />
VOL 1 M37-A2 22 7 M37-A May<br />
2002<br />
VOL 9 EP21-A 23 20 EP21-P April<br />
2003<br />
STATUS<br />
Approved<br />
Guideline<br />
2 nd Edition<br />
Approved<br />
Guideline<br />
TITLE<br />
Development of in vitro susceptibility testing criteria &<br />
QC parameters for vet antimicrobial agents<br />
Estimation of Total Analytical Error for Clinical<br />
Methods<br />
VOL 5 EP14-A2 25 1 EP14-A January<br />
2005<br />
Approved Guideline<br />
– 2 nd Edition<br />
Evaluation of Matrix Effects<br />
VOL 1 EP5-A2 24 25 EP5-A Aug<br />
2004<br />
VOL 6 EP6-A 23 16 EP6-P2 April<br />
2003<br />
VOL 5 H52-A 21 26 H52-P Dec<br />
2001<br />
Approved<br />
Guideline – 2 nd<br />
Edition<br />
Approved<br />
Guideline<br />
Approved Guideline<br />
Evaluation of Precision Performance of Quantitative<br />
Measurement Methods<br />
Evaluation of the Linearity of Quantitative Analytical<br />
Methods<br />
Fetal Red Cell Detection<br />
VOL 10 GP20-A2 23 27 GP20-A Oct 2003 Approved Guideline<br />
2 nd Edition<br />
Fine Needle Aspiration Biopsy (FNAB) Techniques<br />
VOL 11 I/LA24-A 24 26 I/LA24-P Aug<br />
2004<br />
VOL 9 MM7-A 24 5 MM7-P JAN<br />
2004<br />
VOL 6 C25-A 17 3 C25-T JAN<br />
1997<br />
VOL 5 C43-A 22 22 C43-P Nov<br />
2002<br />
Approved Guideline<br />
Approved Guideline<br />
Approved Guideline<br />
Approved Guideline<br />
Fluorescence Calibration and Quantitative<br />
Measurement of Fluorescence Intensity<br />
Fluorescence In Situ Hybridization (FISH) Methods for<br />
Medical Genetics<br />
Fractional Oxyhemoglobin, Oxygen Content and<br />
Saturation, and Related Quantities in Blood:<br />
Terminology, Measurement, and Reporting<br />
Gas Chromatography/Mass Spectometry (GC/MS)<br />
Confirmation of Drugs<br />
4
CLSI UPDATED February 8, 2006<br />
Alphabetical Listing<br />
LOCATION Abbreviation Vol No Replaces Status<br />
Date<br />
VOL 1 AST4-A2 25 12 AST4-A May<br />
2005<br />
VOL 8 C44-A 22 25 C44-P Dec<br />
2002<br />
STATUS<br />
Approved<br />
Guideline<br />
Approved Guideline<br />
TITLE<br />
Glucose Monitoring in Settings Without Laboratory<br />
Support<br />
Harmonization of Glycohemoglobin Measurements<br />
VOL 4 C28-A2 20 13 C28-A Jun 00 Approved Guideline<br />
2 nd Edition<br />
How to define and determine reference intervals in<br />
the clinical lab approved guideline.<br />
VOL 9 MM2-A2 22 12 MM2-A Aug<br />
2002<br />
Approved Guideline -<br />
2 nd Edition<br />
Immunoglobulin and T-Cell receptor gene<br />
rearrangement assays<br />
VOL 7 D12-A2 13 14 1993 Approved Guideline Immunoprecipitin analyses; Procedures for evaluating<br />
the performance of materials<br />
VOL 8 EP7-A2 25 27 EP7-A2 Nov<br />
2005<br />
Approved Guideline<br />
2 nd edition<br />
Interference Testing in Clinical Chemistry<br />
VOL 4 C31-A2 15 20 C31-A Jun 2001 Approved Guideline<br />
2 nd edition<br />
Ionized calcium determinations: Precollection<br />
variables, specimen choice, collection, & handling.<br />
VOL 1 AUTO2-A2 25 29 AUTO2-A Dec<br />
2005<br />
Approved Standard<br />
2 nd edition<br />
Lab automation: Barcodes for specimen container<br />
identification<br />
VOL 2 AUTO3-P 18 18 Dec-98 Lab automation: Communication with automated clinical<br />
laboratory systems instrument devices and information<br />
systems<br />
5
CLSI UPDATED February 8, 2006<br />
LOCATION Abbreviation Vol No Replaces Status<br />
Date<br />
STATUS<br />
TITLE<br />
VOL 2 AUTO5-P 19 23 Oct-99 Proposed Standard Lab automation electromechanical interfaces<br />
VOL 2 AUTO4-P 19 22 Oct-99 Proposed Standard Lab automation: Systems operational requirements,<br />
characteristics, & information elements<br />
VOL 4 M15-A 20 12 M15-T Jun 00 Approved Guideline Lab diagnosis of bloodborne parasitic diseases<br />
VOL 10 AUTO7-A 24 20 AUTO7-P June<br />
2004<br />
Approved Standard<br />
VOL 8 GP18-A 18 3 GP18-P APR 98 Approved Guideline Laboratory Design<br />
VOL 5 GP19-A2 23 4 GP19-A Feb<br />
2003<br />
Approved Guideline<br />
VOL 13 EP13-R 15 8 Aug<br />
1995<br />
VOL 12 I/LA25-A 24 39 I/LA25-P Dec<br />
2004<br />
Report<br />
Approved Standard<br />
Laboratory Automation: Data Content for Specimen<br />
Identification<br />
Laboratory Instruments and Data Management<br />
Systems: Design of Software User Interfaces and<br />
End-User Software Systems Validation, Operation and<br />
Monitoring<br />
Laboratory Statistics<br />
Maternal serum screening<br />
VOL 11 C45-A 24 31 C45-P Oct 2004 Approved Guideline Measurement of Free Thyroid Hormones<br />
VOL 10 ISO 15189 Jul 2003 Corrected Version Medical Laboratories – Particular requirements for<br />
quality and competence<br />
VOL 6 EP9-A2 22 19 EP9-A Sept<br />
2002<br />
Approved Guideline<br />
2 nd Edition<br />
Method comparison and bias estimation using patient<br />
samples<br />
VOL 10 M44-A 24 15 M44-P 5/04 Approved Guideline Method for Antifungal Disk Diffusion Susceptibility<br />
Testing of Yeasts; Approved Guideline<br />
6
CLSI Updated February 8, 2006<br />
Alphabetical Listing<br />
LOCATION Abbreviation Vol No Replaces Status STATUS<br />
TITLE<br />
Date<br />
VOL 3 M21-A 19 17 M21-T Sep-99 Approved Guideline Methodology for the serum bactericidal test<br />
VOL 9 M42-R 23 19 April<br />
2003<br />
Report Published<br />
VOL 6 M11-A6 24 2 M11-A5 Jan<br />
2004<br />
VOL 3 M7-A7 26 2 M7-A6 Jan<br />
2006<br />
Approved Standard<br />
6 th Edition<br />
Approved Standard<br />
7 th Edition<br />
Methods for Antimicrobial Disk Susceptibility Testing<br />
of Bacteria Isolated from Aquatic Animals.<br />
Methods for Antimicrobial Susceptibility Testing of<br />
Anaerobic Bacteria<br />
Methods for Antimicrobial Susceptibility Tests for<br />
Bacteria That Grow Aerobically<br />
VOL 3 M26-A 19 18 M26-T Sep-99 Approved Guideline Methods for determining bactericidal activity of<br />
antimicrobial agents<br />
VOL 8 H44-A2 24 8 H44-A FEB<br />
2004<br />
VOL 10 GP28-A 25 7 GP28-P Feb<br />
2005<br />
Approved Guideline –<br />
2 ND Edition<br />
Approved Guideline<br />
Methods for Reticulocyte Counting ( Automated Blood<br />
Cell Counters, Flow Cytometry and Supravital Dyes)<br />
Microwave Device Used in the Clinical Laboratory<br />
VOL 5 MM1-A 20 7 MM1-P Apr Approved Guideline Molecular diagnostic methods for genetic diseases<br />
00<br />
VOL 8 MM3-A 15 22 MM3-P 1995 Approved Guideline Molecular Diagnostic Methods for Infectious Diseases<br />
VOL 3 GP23-A 19 14 GP23-P Aug-<br />
99<br />
VOL 12 MM9-A 24 40 MM9-P Dec<br />
2004<br />
Approved Guideline<br />
Approved Guideline<br />
Non gynecologic cytologic. specimens collection &<br />
cytoprepatory techniques<br />
Nucleic Acid Sequencing Methods in Diagnostic<br />
Laboratory Medicine<br />
VOL 9 MM5-A 23 17 MM5-P April<br />
2003<br />
VOL 7 H47-A 16 3 H28T &H29T JUN<br />
96<br />
VOL 7 GP15-A2 21 17 GP15-A Nov<br />
2001<br />
Approved Guideline<br />
Approved Guideline<br />
Approved Guideline –<br />
2 nd Edition<br />
Nucleic Acid Amplification Assays for Molecular<br />
Hematopathology<br />
One stage prothrombin time (PT) test and activated<br />
partial thromboplastin time (APTT)<br />
Papincolaou technique<br />
7
CLSI Updated February 8, 2006<br />
Alphabetical Listing<br />
LOCATION Abbreviation Vol No Replaces Statu<br />
s<br />
Date<br />
VOL 5 H26-A 16 12 H26-P DEC<br />
96<br />
STATUS<br />
Approved Standard<br />
TITLE<br />
Performance Goals for the Internal Quality Control of<br />
Multichannel Hematology Analyzers<br />
VOL 11 I/LA26-A 24 29 I/LA26-P Oct<br />
2004<br />
Approved Guideline<br />
Performance of Single Cell Immune Response<br />
Assays<br />
VOL 12 H45-A2 25 15 H45-A June<br />
2005<br />
VOL 3 M31-A2 22 6 M31-A May<br />
2002<br />
VOL 5 M2-A9 26 1 M2-A8 Jan<br />
2006<br />
VOL 3 M100-S12 22 1 M100-S11 Jan<br />
2002<br />
VOL 6 M100-S6 15 14 M100-S5 Dec<br />
1995<br />
VOL 10 M100-S15 25 1 M100-S14 JAN<br />
2005<br />
VOL 13 M100-S16 26 3 M100-S15 Jan<br />
2006<br />
Approved Guideline – 2 nd<br />
Edition<br />
Approved Standard<br />
2 nd Edition<br />
Approved Standard<br />
9 th Edition<br />
12 th INFORMATIONAL<br />
SUPPLEMENT<br />
6 th Informational<br />
Supplement<br />
15 th Informational<br />
Supplement<br />
16 th Informational<br />
Supplement<br />
Performance of the Bleeding Time Test<br />
Performance standards for antimicrobial disk &<br />
dilution susceptibility tests for bacteria isolated from<br />
animals<br />
Performance Standards of Antimicrobial Disk<br />
Susceptibility Tests<br />
Performance standards for antimicrobial susceptibility<br />
testing<br />
Performance Standards for Antimicrobial Susceptibility<br />
Testing<br />
Performance Standards for Antimicrobial Susceptibility<br />
Testing – 15 th Informational Supplement<br />
Performance Standards for Antimicrobial Susceptibility<br />
Testing –16 th Informational Supplement<br />
CD Case I POCT1-A Approved Standard Point of care connectivity<br />
VOL 8 C30-A2 22 17 C30-A Aug Approved Guideline Point-of-Care Blood Glucose Testing in Acute and<br />
2002 2 nd Edition<br />
Chronic Care Facilities<br />
VOL 1 AST2-A 15 2 AST2-P Approved<br />
Point of care invitro diagnostic (IVD) testing<br />
Guideline<br />
VOL 11 H49-A 24 23 H49-P JUL<br />
2004<br />
Approved Guideline<br />
Point-of-Care Monitoring of Anticoagulation Therapy<br />
VOL 6 EP10-A2 18 6 EP10-A Dec<br />
2002<br />
Approved Guideline –<br />
2 nd Edition<br />
Preliminary Evaluation of Quantitative Clinical<br />
Laboratory Methods<br />
8
CLSI Updated February 8, 2006<br />
Alphabetical Listing<br />
LOCATION Abbreviation Vol No Replaces Statu<br />
s<br />
Date<br />
VOL 5 C3-P4 25 13 C3-A3 June<br />
2005<br />
STATUS<br />
Proposed Guideline<br />
4 th Edition<br />
TITLE<br />
Preparation & testing of Reagent Water in the Clinical<br />
Laboratory<br />
VOL 3 C37-A 19 25 C37-P Nov-<br />
99<br />
Approved Guideline<br />
Preparation & validation of commutable frozen human<br />
serum pools as secondary reference materials for<br />
cholesterol measurement<br />
VOL 5 H7-A3 20 18 H7-A2 Approved Standard Procedure for Determining Packed Cell Volume by the<br />
Microhematocrit Method<br />
VOL 7 * H3-A5 23 32 H3-A4 DEC<br />
2003<br />
VOL 7 H30-A2 21 18 H30-A Nov<br />
2001<br />
Approved Standard –<br />
5 th Edition<br />
Approved Guideline – 2 nd<br />
Edition<br />
Procedure for the Collection of Diagnostic Blood<br />
Specimens by Venipuncture.<br />
Procedure for the determination of fibrinogen in<br />
plasma<br />
VOL 3 * H4-A4 19 16 Consolidates<br />
H4-A3 &<br />
H14-A2<br />
Sep-<br />
99<br />
VOL 13 H54-A 25 23 H54-P Aug<br />
2005<br />
Approved Standard<br />
Approved Guideline<br />
Procedures and devices for collection of diagnostic<br />
blood specimens by skin puncture<br />
Procedures for Validation of INR and Local Calibration of<br />
PT/INR Systems<br />
VOL 3 H11-A4 24 28 H11-A3 Sept<br />
2004<br />
VOL 2 H 18-A3 24 38 H18-A2 Nov<br />
2004<br />
VOL 12 M28-A2 25 16 M28-A June<br />
2005<br />
Approved Standard – 4 th<br />
Edition<br />
Approved Guideline –<br />
2 nd Edition<br />
Approved Guideline –<br />
2 nd Edition<br />
Procedures for the collection of arterial blood<br />
specimens<br />
Procedures for the handling and processing of blood<br />
specimens<br />
Procedures for the Recovery and Identification of<br />
Parasites from the Intestinal Tract<br />
VOL 11 MM14-A 25 24 MM14-P Aug<br />
2005<br />
Approved Guideline<br />
Proficiency Testing (External Quality Assessment) for<br />
Molecular Methods<br />
9
CLSI Updated February 8, 2006<br />
Alphabetical Listing<br />
LOCATION Abbreviation Vol No Replaces Statu<br />
s<br />
Date<br />
VOL 6 M29-A3 25 10 M29-A2 March<br />
2005<br />
STATUS<br />
Approved Guideline<br />
- 3rd Edition<br />
TITLE<br />
Protection of laboratory workers from occupationally<br />
acquired infections<br />
VOL 11 EP17-A 24 34 EP17-P Oct<br />
2004<br />
Approved Guideline<br />
Protocols for Determination of Limits of Detection and<br />
Limits of Quantitation<br />
VOL 13 M6-A2 26 6 M6-A Jan<br />
2006<br />
Approved Standard -<br />
2 nd Edition<br />
Protocols for Evaluating Dehydrated Mueller-Hinton<br />
Agar<br />
VOL 6 HS2-A 23 5 HS2-P Feb<br />
2003<br />
VOL 12 HS3-A 25 5 HS3-P Jan<br />
2005<br />
Approved Guideline<br />
Approved Guideline<br />
Provider-Performed Microscopy Testing<br />
Pulse Oximetry<br />
VOL 5 M22-P2 23 22 M22-A Aug<br />
2003<br />
VOL 4 I/LA2-A 16 11 I/LA2-T Dec-<br />
96<br />
VOL 10 M40-A 23 34 M40-P Dec<br />
2003<br />
VOL 3 MM4-P 17 10 Dec<br />
99<br />
VOL 4 EP18-A 22 28 EP18-P Dec<br />
2002<br />
VOL 10 MM6-A 23 28 MM6-P Oct<br />
2003<br />
VOL 8 M38-A 22 16 M38-P AUG<br />
2002<br />
VOL 8 M27-A2 22 15 M27-A AUG<br />
2002<br />
Proposed Standard –<br />
2 nd Edition<br />
Approved Guideline<br />
Approved Standard<br />
Approved Guideline<br />
Approved Guideline<br />
Approved Guideline<br />
Approved Standard<br />
Approved Standard –<br />
2nd Edition<br />
Quality Assurance for Commercially Prepared<br />
Microbiological Culture Media<br />
Quality assurance for the indirect immunofluorescence<br />
test for autoantibodies to nuclear antigen(if-ana)<br />
Quality Control of Microbiological Transport Systems<br />
Quality for immunocytochemistry<br />
Quality management for unit-use testing<br />
Quantitative Molecular Methods for Infectious Diseases<br />
Reference Method for Broth Dilution Antifungal<br />
Susceptibility Testing of Filamentous Fungi<br />
Reference Method for Broth Dilution Antifungal Susceptibility<br />
Testing of Yeasts<br />
10
CLSI Updated February 8, 2006<br />
Alphabetical Listing<br />
LOCATION Abbreviation Vol No Replaces Statu<br />
s<br />
Date<br />
11<br />
VOL 3 GP9-A 18 15 GP9-T Nov-<br />
98<br />
STATUS<br />
VOL 4 H2-A4 20 27 H2-A3<br />
Approved Standard –<br />
Dec0 4th Edition<br />
0<br />
VOL 4 H15-A3 20 28 H15-A2 Dec0 Approved Standard –<br />
0 3rd Edition<br />
Approved Guideline<br />
VOL 11 LIS2-A 24 33 LIS2-A Oct<br />
2004<br />
VOL 6 I/LA18-A2 21 15 I/LA18-A Sep<br />
2001<br />
Approved Standard –<br />
2nd Edition<br />
Approved Guideline<br />
TITLE<br />
Reference & selected procedure for the erythrocyte<br />
sedimentation rate (ESR) test<br />
Reference & selected procedures for the quantitative<br />
determination of hemoglobin in blood<br />
Selecting and evaluating a referral laboratory<br />
Specification for Transferring Information Between<br />
Clinical Laboratory Instruments and Information<br />
Systems<br />
Specifications for Immunological Testing for Infectious<br />
Diseases<br />
VOL 5 C29-A2 20 17 C29-A Approved Standard Standardization of Sodium & Potassium Ion-Selective<br />
Electrode Systems to the Flame Photometric<br />
Reference Method<br />
VOL 1 C24-A2 11 8 C24-A Feb-<br />
99<br />
VOL 11 HS6-A 24 32 HS6-P Oct<br />
2004<br />
VOL 4 C34-A2 20 14 C34-A Jun<br />
00<br />
VOL 7 M24-A 23 18 M24-T2 April<br />
2003<br />
Approved<br />
Guideline<br />
Approved Guideline<br />
Approved Guideline<br />
2nd Edition<br />
Approved Standard<br />
Statistical Q.C. for quantitative measurements<br />
principles & definitions<br />
Studies to Evaluate Patient Outcomes<br />
Sweat testing sample collection & quantitative analysis<br />
Susceptibility Testing of Mycobacteria, Nocardia, and<br />
Other Aerobic Actinomycetes<br />
VOL 5 NRSCL13-A 20 21 NRSCL 13-P Approved Guideline The Reference System for the Clinical Laboratory:<br />
Criteria for Development and Credentialing of Methods<br />
and Materials for Harmonization of Results<br />
VOL 10 MM6-A 23 28 MM6-P Oct<br />
2003<br />
VOL 8 M38-A 22 16 M38-P AUG<br />
2002<br />
VOL 8 M27-A2 22 15 M27-A AUG<br />
2002<br />
Approved Guideline<br />
Approved Standard<br />
Approved Standard –<br />
2 nd Edition<br />
Quantitative Molecular Methods for Infectious<br />
Diseases<br />
Reference Method for Broth Dilution Antifungal<br />
Susceptibility Testing of Filamentous Fungi<br />
Reference Method for Broth Dilution Antifungal<br />
Susceptibility Testing of Yeasts
CLSI Updated February 8, 2006<br />
Alphabetical Listing<br />
VOL 4 H2-A4 20 27 H2-A3<br />
Approved Standard – Reference & selected procedure for the erythrocyte<br />
Dec0 4 th Edition<br />
sedimentation rate (ESR) test<br />
0<br />
LOCATION Abbreviation Vol No Replaces Statu STATUS<br />
TITLE<br />
s<br />
Date<br />
VOL 4 H15-A3 20 28 H15-A2 Dec0<br />
0<br />
VOL 3 GP9-A 18 15 GP9-T Nov-<br />
98<br />
VOL 11 LIS2-A 24 33 LIS2-A Oct<br />
2004<br />
VOL 6 I/LA18-A2 21 15 I/LA18-A Sep<br />
2001<br />
Approved Standard –<br />
3 rd Edition<br />
Approved Guideline<br />
Approved Standard –<br />
2 nd Edition<br />
Approved Guideline<br />
Reference & selected procedures for the quantitative<br />
determination of hemoglobin in blood<br />
Selecting and evaluating a referral laboratory<br />
Specification for Transferring Information Between<br />
Clinical Laboratory Instruments and Information<br />
Systems<br />
Specifications for Immunological Testing for Infectious<br />
Diseases<br />
VOL 5 C29-A2 20 17 C29-A Approved Standard Standardization of Sodium & Potassium Ion-Selective<br />
Electrode Systems to the Flame Photometric<br />
Reference Method<br />
VOL 1 C24-A2 11 8 C24-A Feb-<br />
99<br />
VOL 11 HS6-A 24 32 HS6-P Oct<br />
2004<br />
VOL 4 C34-A2 20 14 C34-A Jun<br />
00<br />
VOL 7 M24-A 23 18 M24-T2 April<br />
2003<br />
Approved<br />
Guideline<br />
Approved Guideline<br />
Approved Guideline<br />
2 nd Edition<br />
Approved Standard<br />
Statistical Q.C. for quantitative measurements<br />
principles & definitions<br />
Studies to Evaluate Patient Outcomes<br />
Sweat testing sample collection & quantitative analysis<br />
Susceptibility Testing of Mycobacteria, Nocardia, and<br />
Other Aerobic Actinomycetes<br />
VOL 5 NRSCL13-A 20 21 NRSCL 13-P Approved Guideline The Reference System for the Clinical Laboratory: Criteria<br />
for Development and Credentialing of Methods and Materials<br />
for Harmonization of Results<br />
VOL 5 GP21-A2 24 14 GP21-A APR<br />
2004<br />
Approved Guideline-2 nd<br />
Edition<br />
Training and Competence Assessment<br />
VOL 10 H1-A5 23 33 H1-A4 Dec<br />
2003<br />
Approved Standard –<br />
5 th Edition<br />
Tubes and Additives for Venous Blood Specimen<br />
Collection<br />
12
CLSI Updated February 8, 2006<br />
VOL 5<br />
Alphabetical Listing<br />
GP16-A2 15 15 GP16-A NOV<br />
2001<br />
Approved Guideline –<br />
2 ND EDITION<br />
Urinalysis and Collection, Transportation, and<br />
Preservation of Urine Specimens<br />
LOCATION Abbreviation Vol No Replaces Statu<br />
s<br />
Date<br />
VOL 1 T/DMB-A 19 6 T/DMB-P Feb<br />
99<br />
VOL 7 EP12-A 22 14 EP12-P Aug<br />
2002<br />
STATUS<br />
Approved<br />
Guideline<br />
Approved Guideline<br />
TITLE<br />
Urine drug testing in the clinical lab<br />
User protocol for evaluation of qualitative test<br />
performance<br />
VOL 7 EP15-A2 25 17 EP15-A June<br />
2005<br />
Approved Guideline-<br />
2 nd Edition<br />
User verification of Performance for Precision and<br />
Trueness<br />
VOL 1 GP27-A 19 15 GP22-P Aug<br />
99<br />
Approved<br />
Guideline<br />
Using proficiency test (PT) to improve the clinical<br />
laboratory<br />
VOL 5<br />
M34-A 20 20 M34-P Approved Guideline Western Blot Assay for Antibodies to Borrelia<br />
burgdorferi<br />
* Video is also available through CQI Office<br />
13