experiment 9 : separation of a mixture by paper chromatography
experiment 9 : separation of a mixture by paper chromatography
experiment 9 : separation of a mixture by paper chromatography
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
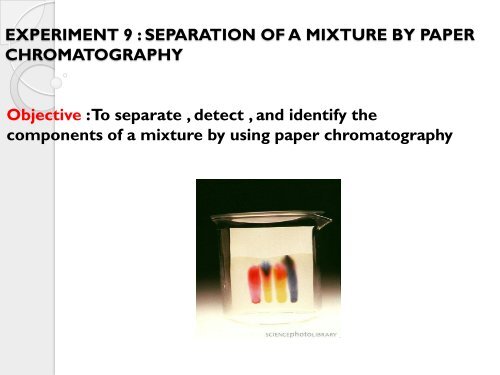
EXPERIMENT 9 : SEPARATION OF A MIXTURE BY PAPER<br />
CHROMATOGRAPHY<br />
Objective : To separate , detect , and identify the<br />
components <strong>of</strong> a <strong>mixture</strong> <strong>by</strong> using <strong>paper</strong> <strong>chromatography</strong>
Introduction<br />
Paper <strong>chromatography</strong> is a method <strong>of</strong><br />
separating <strong>mixture</strong>s <strong>by</strong> using a piece <strong>of</strong><br />
absorbent <strong>paper</strong>. In this process, the<br />
solution to be separated is placed on a<br />
piece <strong>of</strong> dry filter <strong>paper</strong>. This is the<br />
stationary phase. A solvent (the moving<br />
phase) is allowed to travel across the<br />
<strong>paper</strong> <strong>by</strong> capillary action. As the solvent<br />
front moves, the components <strong>of</strong> the<br />
<strong>mixture</strong> separate.
R f values<br />
The distance traveled <strong>by</strong> a component <strong>of</strong> a spot with<br />
respect to the distance traveled <strong>by</strong> the pure liquid is a<br />
measure <strong>of</strong> that component's competitive affinities for<br />
the stationary and mobile phases. We define the<br />
component's Rf (retention factor) value I those terms:<br />
For example, if one component <strong>of</strong> a <strong>mixture</strong> travelled 9.6<br />
cm from the base line while the solvent had travelled<br />
12.0 cm, then the Rf value for that component is:
Chemical Used<br />
• Fe 3+ solution .<br />
• Cu 2+ solution .<br />
• Ni 2+ solution .<br />
• Mixture ( 90% acetone<br />
and 10% 6 M HCl .<br />
• 15 M ammonia .<br />
• Dimethylglyoxime.
Procedure<br />
1. In a piece <strong>of</strong> <strong>chromatography</strong> filter <strong>paper</strong> draw 1 line 2 cm from<br />
one <strong>of</strong> the longer edges. Mark the line with a pencil dot at six<br />
places
2. Fill your capillary pipet <strong>by</strong> dipping the end into the solutions<br />
<strong>of</strong> ions( Cu 2+ , Fe 3+ , and Ni 2+ ) , Known solution <strong>of</strong> the three<br />
ions, and unknown solution . Allow the solution to rise <strong>by</strong><br />
capillary action.<br />
3. Apply a single drop <strong>of</strong> the solutions in to the filter <strong>paper</strong> .
4. Pour the <strong>mixture</strong> in to a depth <strong>of</strong> 1 cm (about 20 mL) in<br />
the bottom <strong>of</strong> a 600-mL beaker .<br />
5. Fold the filter <strong>paper</strong> and staple to make a cylinder shape<br />
as shown in the figure below and place it in the beaker.<br />
6. Cover the beaker carefully with a watch glass. Do not<br />
disturb the beaker while the chromatograms are developing<br />
7 . When the solvent front has nearly reached the top <strong>of</strong> the<br />
<strong>paper</strong>, carefully remove the watch glass and the <strong>paper</strong>. Place<br />
the wet <strong>paper</strong> on the <strong>paper</strong> towel and immediately mark the<br />
solvent front with a pencil. Allow the filter <strong>paper</strong> to dry.
Tips<br />
•The 2-cm line should be above the surface <strong>of</strong> the liquid Be<br />
certain that the spots on<br />
•The <strong>paper</strong> are completely dry before you place the <strong>paper</strong> in<br />
the beaker .<br />
• Make sure that the <strong>paper</strong> does not touch the wall <strong>of</strong> the<br />
beaker and that the bottom <strong>of</strong> the <strong>paper</strong> is resting in the<br />
solution.
Observation:<br />
1. Fe 3+ in water imparts a red-brown or rust color and thus will<br />
produce a rust-colored band on the <strong>paper</strong> with out any<br />
development. As shown im the figure below.<br />
1. Fe 3+ Yellow<br />
color
2. To detect the second ion (Cu 2+ ) :<br />
IN THE HOOD, pour about 5 mL <strong>of</strong> 15 M ammonia into a clean,<br />
shallow dish and rest the filter <strong>paper</strong> on the top <strong>of</strong> the dish. Do not<br />
permit the <strong>paper</strong> to dip into the solution.<br />
What color develops ? Blue spots<br />
What ions dos this indicate ? Cu 2+<br />
Cu 2+ is blue, the color is faint and not easily detected, especially<br />
if copper is present in small amounts. In aqueous solution,<br />
however, Cu 2+ reacts with NH 3 (from ammonium hydroxide) to<br />
form a complex ion, [Cu(NH 3 ) 4 ] 2+ , which is deep blue and<br />
therefore readily observed<br />
Cu
3. To detect the third ion, dip a new piece <strong>of</strong> filter <strong>paper</strong> into<br />
a solution <strong>of</strong> dimethylglyoxime; then, using the new piece as<br />
a brush, paint the test filter <strong>paper</strong>.<br />
What color develops ? Pink spot<br />
What ions does this indicate ? Ni 2+<br />
Ni
4. Analyze the <strong>paper</strong> , notice that the <strong>mixture</strong> contains<br />
three spots that indicate the three ions, and the<br />
unknown contains two spots .<br />
Fe 3+<br />
Trace Cu 2+<br />
Cu 2+<br />
Unknown (Fe 3+ &<br />
Ni 2+ )<br />
Unknown<br />
(Fe 3+ , Cu 2+ &<br />
Ni 2+ )<br />
Ni 2+
5. Measure distances from the start line to the solvent front and<br />
to the middle <strong>of</strong> each spot.(R f ) Write down results in<br />
your work sheet.
• Understanding the results:<br />
The number <strong>of</strong> the spots or bands tells<br />
you how many compounds are in your<br />
substance. Their color and the distance<br />
they traveled might help you to identify<br />
those compounds.
REPORT SHEET<br />
1. Color <strong>of</strong> spots: Cu 2+ ________ Ni 2+ _________ Fe 3+ ___________<br />
2. Ion requiring no development ___________<br />
3. Ammonia develops a ___________ color.<br />
4. The ion that causes the color <strong>of</strong> (3) is _________<br />
5. Dimethylglyoxime develops a ______________ color.<br />
6. The ion that causes the color <strong>of</strong> (5) is __________<br />
7. Ring front distances for knowns are: solvent: _______ mm;<br />
Cu 2+ _________ mm; Ni 2+ __________ mm ; Fe 3+ _________ mm.<br />
8. R f values for knowns: Cu 2+ ________ ; Ni 2+ ________ ; Fe 3+ ________<br />
9. Unknown label ________<br />
10. Ring front distances for unknowns:<br />
__________ mm ; __________ mm ; __________ mm.<br />
11. R f values for unknowns:<br />
____________ mm ; ____________ mm ; ____________ mm ; .<br />
12. Ions present in unknown __________________