exotic nuclei structure and reaction noyaux exotiques ... - IPN - IN2P3
exotic nuclei structure and reaction noyaux exotiques ... - IPN - IN2P3
exotic nuclei structure and reaction noyaux exotiques ... - IPN - IN2P3
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
(D 0<br />
/D)-1<br />
ln<br />
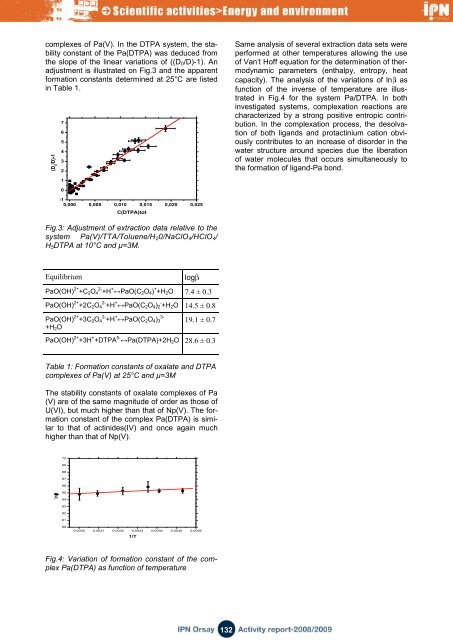
complexes of Pa(V). In the DTPA system, the stability<br />
constant of the Pa(DTPA) was deduced from<br />
the slope of the linear variations of ((D 0 /D)-1). An<br />
adjustment is illustrated on Fig.3 <strong>and</strong> the apparent<br />
formation constants determined at 25°C are listed<br />
in Table 1.<br />
7<br />
6<br />
5<br />
4<br />
3<br />
2<br />
Same analysis of several extraction data sets were<br />
performed at other temperatures allowing the use<br />
of Van’t Hoff equation for the determination of thermodynamic<br />
parameters (enthalpy, entropy, heat<br />
capacity). The analysis of the variations of ln as<br />
function of the inverse of temperature are illustrated<br />
in Fig.4 for the system Pa/DTPA. In both<br />
investigated systems, complexation <strong>reaction</strong>s are<br />
characterized by a strong positive entropic contribution.<br />
In the complexation process, the desolvation<br />
of both lig<strong>and</strong>s <strong>and</strong> protactinium cation obviously<br />
contributes to an increase of disorder in the<br />
water <strong>structure</strong> around species due the liberation<br />
of water molecules that occurs simultaneously to<br />
the formation of lig<strong>and</strong>-Pa bond.<br />
1<br />
0<br />
-1<br />
0,000 0,005 0,010 0,015 0,020 0,025<br />
C(DTPA)tot<br />
Fig.3: Adjustment of extraction data relative to the<br />
system Pa(V)/TTA/Toluene/H 2 0/NaClO 4 /HClO 4 /<br />
H 5 DTPA at 10°C <strong>and</strong> µ=3M.<br />
Equilibrium<br />
log<br />
PaO(OH) 2+ +C 2 O 4 2- +H + ↔PaO(C 2 O 4 ) + +H 2 O 7.4 ± 0.3<br />
PaO(OH) 2+ +2C 2 O 4 2- +H + ↔PaO(C 2 O 4 ) 2 - +H 2 O 14.5 ± 0.8<br />
PaO(OH) 2+ +3C 2 O 4 2- +H + ↔PaO(C 2 O 4 ) 3<br />
3-<br />
+H 2 O<br />
19.1 ± 0.7<br />
PaO(OH) 2+ +3H + +DTPA 5- ↔Pa(DTPA)+2H 2 O 28.6 ± 0.3<br />
Table 1: Formation constants of oxalate <strong>and</strong> DTPA<br />
complexes of Pa(V) at 25°C <strong>and</strong> µ=3M<br />
The stability constants of oxalate complexes of Pa<br />
(V) are of the same magnitude of order as those of<br />
U(VI), but much higher than that of Np(V). The formation<br />
constant of the complex Pa(DTPA) is similar<br />
to that of actinides(IV) <strong>and</strong> once again much<br />
higher than that of Np(V).<br />
70<br />
69<br />
68<br />
67<br />
66<br />
65<br />
64<br />
63<br />
62<br />
61<br />
60<br />
0,0030 0,0031 0,0032 0,0033 0,0034 0,0035 0,0036<br />
1/T<br />
Fig.4: Variation of formation constant of the complex<br />
Pa(DTPA) as function of temperature<br />
132