Solubility Practice a Curvy Subject
Solubility Practice a Curvy Subject
Solubility Practice a Curvy Subject
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Teacher page<br />
Chemistry I<br />
EQ: Why do some substances dissolve in water<br />
while others do not?<br />
<strong>Solubility</strong> <strong>Practice</strong> a<br />
<strong>Curvy</strong> <strong>Subject</strong><br />
Enduring Understanding<br />
Resource use is determined by properties and availability.<br />
Broad Brush Knowledge Concentrations, Measurement, Colligative Properties,<br />
Saturation<br />
Concepts Important to Know and Understand<br />
Chemical Quantities, Interactions of Matter<br />
Core Objectives: Describe the nature of water and aqueous solutions:<br />
Describe the structure of the water molecule and explain how its polarity affects<br />
its properties such as surface tension, solubility, boiling point, freezing point, and<br />
specific heat. Describe solutions in terms of solute, solvent, solvation, degrees of<br />
saturation, relative concentration, and colligative properties.<br />
TEKS 12A demonstrate and explain effects of temperature and the nature of solid solutes on the solubility of solids.<br />
13 The student knows relationships among the concentration, electrical conductivity, and colligative properties of a solution. The student is<br />
expected to: A: compare unsaturated, saturated, and supersaturated solutions B: interpret relationships among ionic and covalent<br />
compounds, electrical conductivity, and colligative properties of water, 2D: organize, analyze, evaluate, make inferences, and predict trends<br />
from data; 2E: communicate valid conclusions.<br />
TEACHER MANAGEMENT<br />
Estimated Time: 20-30 minutes could be used as homework in Unit I Section C.1.<br />
Materials: Copies for all students<br />
Teacher Prep: None<br />
Safety Precautions: None<br />
PRACTICE<br />
PURPOSE: to practice reading and interpreting solubility graphs.<br />
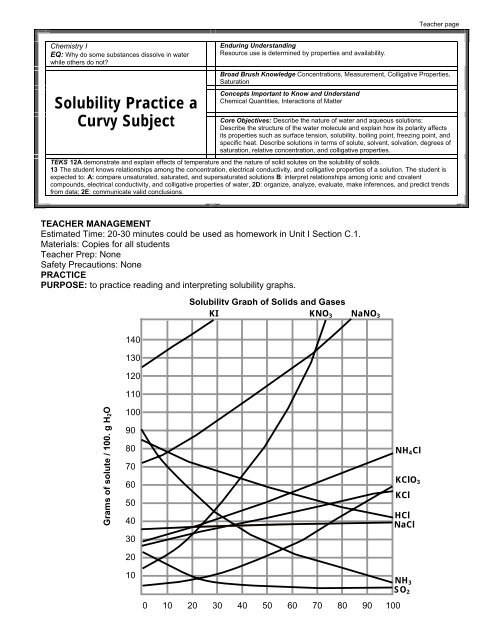
<strong>Solubility</strong> Graph of Solids and Gases<br />
KI KNO 3 NaNO 3<br />
140<br />
130<br />
120<br />
110<br />
Grams of solute / 100. g H2O<br />
100<br />
90<br />
80<br />
70<br />
60<br />
50<br />
40<br />
30<br />
20<br />
10<br />
0 10 20 30 40 50 60 70 80 90 100<br />
NH 4 Cl<br />
KClO 3<br />
KCl<br />
HCl<br />
NaCl<br />
NH 3<br />
SO 2
Teacher Page<br />
KEY<br />
Temperature ( 0 C)<br />
Directions: Answer in complete sentences and show all work for calculations.<br />
1. What relationship exists between solubility and temperature for most of the substances shown?<br />
A direct relationship is shown for most substances, as temperature increases the<br />
solubility of most substances increases.<br />
2. What is the exception?<br />
The exception is gas solubility. Gases are less soluble at higher temperature,<br />
illustrating an indirect relationship<br />
3. What explains why solids become more soluble as temperature increases and why gases become<br />
less soluble? Use the following words to help justify your response: temperature, collisions and<br />
energy.<br />
As temperature increases both speed of the water molecules and the number of<br />
collisions increase. A solid remains dissolved by the continual motion of the eater<br />
molecules. The more energy and motion, the more particles that can remain in<br />
solution. This is especially true for ionic compounds, because water is polar and the<br />
opposite charges help stick the water molecules to the ions. Gases are often nonpolar<br />
and they have very low boiling points, so as temperature increases they tend to “boil<br />
away”. That is why a soda will go flat much faster if it is left in a warm place.<br />
4. Which is more soluble NaNO 3 or KCl? Does the temperature matter? Why or why not?<br />
NaNO 3 because the higher line indicates that more NaNO 3 can be dissolved at ALL<br />
temperatures.<br />
5. How does the line drawn for a particular substance relate to the saturation of a solution of that<br />
substance? Use the following words to help justify your response: saturated, unsaturated,<br />
supersaturated.<br />
The line marks the saturation point of a solute in 100 grams of water. If you have less<br />
grams dissolved than indicated by the line then your solution is unsaturated at that<br />
temperature. If you have more, then your solution is supersaturated at that<br />
temperature.<br />
6. How many grams of NH 4 Cl will dissolve in 100. grams of water at 90. 0 C? 72 grams<br />
7. How many grams of KClO 3 will dissolve in 300. grams of water at 30. 0 C?<br />
300. g of H 2 O X 10. g KClO 3 = 30. g KClO 3<br />
100. g H 2 O<br />
8. How would you make a saturated solution of KNO 3 at 60. 0 C in 50. grams of water?<br />
The graph indicates that that 106 grams of KNO 3 will dissolve in 100. grams of water,<br />
so I need only 106g /2= 53 grams of KNO 3 to make the solution. So I would measure<br />
out 50 grams of water and 53 grams of KNO 3 and mix them together to make the<br />
solution.<br />
9. If you were asked to make a saturated solution of KCl in 100. grams of water, what other piece of<br />
information would you need to before you could start? Why?<br />
You would need to know the temperature of the solution. The saturation point is very<br />
different depending on the temperature<br />
10. If you start with a saturated solution of NH 3 in 100. grams of water at 10. 0 C, how many grams of NH 3<br />
gas would bubble out of solution if you raise the temperature to 80. 0 C?
100. grams of water at 10. 0 C can hold 70. grams of NH 3 .<br />
100. grams of water at 80.0C can hold 14 grams of NH 3 .<br />
So 70. g -14 g = 56 grams of NH 3 will bubble out.<br />
Teacher Page<br />
11. A saturated solution of NaNO 3 was made with 300. grams of water at 40. 0 C. How much NaNO 3<br />
could be recovered by evaporating the solution to dryness?<br />
So 300. grams of water can hold 3 X 106 g = 318 g NaNO 3 will be recovered.<br />
12. A saturated solution KNO 3 in 400. grams of water at 50. 0 C is cooled to 10. 0 C. How much KNO 3 will<br />
come out of solution as crystals?<br />
From the graph 100. grams of water at 50. 0 C can hold 83 g of KNO 3<br />
So 400. grams of water can hold 4 X 83 g = 332 g KNO 3<br />
From the graph 100. grams of water at 10. 0 C can hold 21 g of KNO 3<br />
So 400. grams of water can hold 4 X 21 g = 84 g KNO 3<br />
As the solution is cooled 332 g- 84g = 248 g KNO 3 will form crystals.<br />
13. You start with 200. grams of ice saturated with SO 2 at 0. 0 C. How many grams of SO 2 will bubble out<br />
of solution if you melt the ice and raise the temperature of the water to 80. 0 C?<br />
From the graph 100.g of ice at 0. 0 C can hold 23 g of SO 2<br />
So 200. grams of ice can hold 2 X 24 g = 48 g SO 2<br />
From the graph 100g. of water at 80. 0 C can hold 4 g of SO 2<br />
So 200. grams of water can hold 2 X 4g = 8 g of SO 2<br />
As the solution is heated 48 g – 8 g = 40 g of SO 2 will bubble out.