N2O production in a single stage nitritation/anammox MBBR process
N2O production in a single stage nitritation/anammox MBBR process
N2O production in a single stage nitritation/anammox MBBR process
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
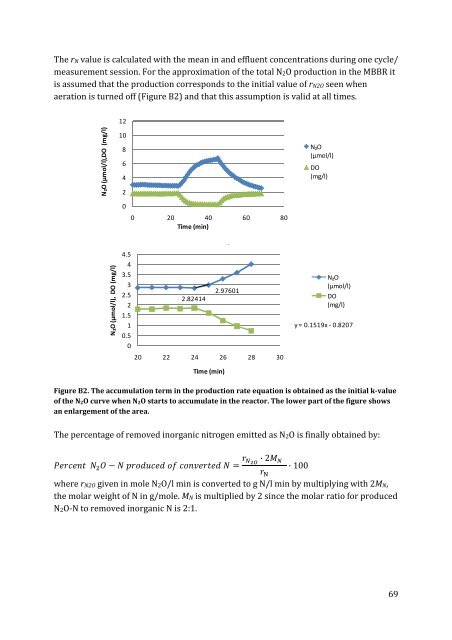
The rN value is calculated with the mean <strong>in</strong> and effluent concentrations dur<strong>in</strong>g one cycle/<br />
measurement session. For the approximation of the total <strong>N2O</strong> <strong>production</strong> <strong>in</strong> the <strong>MBBR</strong> it<br />
is assumed that the <strong>production</strong> corresponds to the <strong>in</strong>itial value of r<strong>N2O</strong> seen when<br />
aeration is turned off (Figure B2) and that this assumption is valid at all times.<br />
12<br />
N₂O (µmol/l),DO (mg/l)<br />
10<br />
8<br />
6<br />
4<br />
2<br />
N₂O<br />
(µmol/l)<br />
DO<br />
(mg/l)<br />
N₂O (µmol/l), DO (mg/l)<br />
0<br />
4.5<br />
4<br />
3.5<br />
3<br />
2.5<br />
2<br />
1.5<br />
1<br />
0.5<br />
0<br />
0 20 40 60 80<br />
Time (m<strong>in</strong>)<br />
Initial N₂O <strong>production</strong><br />
2.82414<br />
2.97601<br />
20 22 24 26 28 30<br />
N₂O<br />
(µmol/l)<br />
DO<br />
(mg/l)<br />
y = 0.1519x - 0.8207<br />
Time (m<strong>in</strong>)<br />
Figure B2. The accumulation term <strong>in</strong> the <strong>production</strong> rate equation is obta<strong>in</strong>ed as the <strong>in</strong>itial k-value<br />
of the N 2O curve when N 2O starts to accumulate <strong>in</strong> the reactor. The lower part of the figure shows<br />
an enlargement of the area.<br />
The percentage of removed <strong>in</strong>organic nitrogen emitted as <strong>N2O</strong> is f<strong>in</strong>ally obta<strong>in</strong>ed by:<br />
· 2 <br />
<br />
· 100<br />
where r<strong>N2O</strong> given <strong>in</strong> mole <strong>N2O</strong>/l m<strong>in</strong> is converted to g N/l m<strong>in</strong> by multiply<strong>in</strong>g with 2MN,<br />
the molar weight of N <strong>in</strong> g/mole. MN is multiplied by 2 s<strong>in</strong>ce the molar ratio for produced<br />
<strong>N2O</strong>-N to removed <strong>in</strong>organic N is 2:1.<br />
69